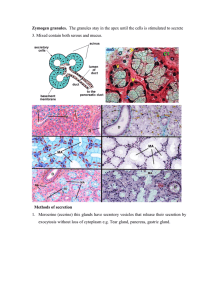
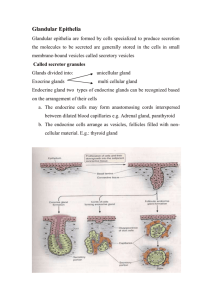
Clinical Implications of Goblet Cells in Dacryoadenosis and Normal Human Lacrimal Glands FREDERICK A. JAKOBIEC, RALPH C. EAGLE JR, MARTIN SELIG, LINA MA, AND CAROL SHIELDS PURPOSE: The purpose of this study was to investigate an enlarged dacryoadenotic lacrimal gland and normal lacrimal glands for the presence of goblet cells (mucocytes). DESIGN: Retrospective clinicopathologic series. METHODS: An enlarged lacrimal gland (dacryoadenosis) without obvious histopathologic alterations was extensively evaluated histochemically, immunohistochemically, and ultrastructurally to detect the presence of goblet cells and to compare the findings with those in five normal lacrimal glands. RESULTS: Granular, zymogen-rich pyramidal acinar cells in normal glands predominated over a previously not reported subpopulation of nongranular, palestaining cells in both dacryoadenotic and normal lacrimal glands. These cells histochemically stained positively with mucicarmine and Alcian blue. Immunohistochemical and electron microscopic evaluations established that there was a displacement or replacement of cytoplasmic gross cystic disease fluid protein-15 and CK 7positive tonofilaments in the pale acinar cells by myriad mucus granules. The goblet cells constituted approximately 2% of the normal acinar cells and 5% of dacryoadenotic acinar cells. A depletion of myoepithelial cells and ectopic intra-acinar ductular cells were also observed in dacryoadenosis. CONCLUSION: Dacryoadenosis is caused by an increase in the number of acini without individual acinar cell hyperplasia. A normal cytologic feature of the lacrimal gland is the presence of acinar goblet cells that had been long overlooked; they are increased in number in dacryoadenosis. Intra-acinar ductular cells and the scattered loss of myoepithelial cells are other abnormalities in dacryoadenosis. The presence of lacrimal gland goblet cells may Accepted for publication Jan 19, 2020. From the David G. Cogan Laboratory of Ophthalmic Pathology and the Department of Ophthalmology (F.A.J., L.M.), Massachusetts Eye and Ear Infirmary and Harvard Medical School, Boston, Massachusetts, USA; Department of Pathology (R.C.E.), Wills Eye Hospital, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, USA; Department of Pathology (M.S.), Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA; and the Ocular Oncology Service (C.S.), Wills Eye Hospital, Philadelphia, Pennsylvania, USA. Inquiries to Frederick A. Jakobiec, David G. Cogan Laboratory of Ophthalmic Pathology, Department of Ophthalmology, Massachusetts Eye and Ear Infirmary, Suite 328, 243 Charles Street, Boston, Massachusetts 02114, USA; e-mail: Fred_Jakobiec@meei.harvard.edu 0002-9394/$36.00 https://doi.org/10.1016/j.ajo.2020.01.029 © 2020 have physiologic implications for the precorneal tear film and its derangements as well as for the histogenesis of mucus-producing carcinomas. (Am J Ophthalmol 2020;213:267–282. Ó 2020 Elsevier Inc. All rights reserved.) S IALADENOSIS IS AN UNUSUAL NON-NEOPLASTIC AND non-inflammatory condition of the salivary glands.1 The term designates a diffuse enlargement without any discernible microscopic abnormality of the involved glandular parenchyma. The parotid is involved most often, followed by the submandibular gland. Based on clinical information and behavior, this disorder is probably acquired rather than congenital. Head and neck pathologists regard the lacrimal gland as a minor salivary gland because of their similar structures, which would imply that a condition like sialadenosis may have an analog in the lacrimal gland. Such a lacrimal gland lesion has not yet been described in the medical literature, nor is it alluded to in the recently published World Health Organization (WHO) Classification of Tumors of The Eye (2018).2 Moreover, the salivary and lacrimal glands share many of the same neoplastic and non-neoplastic lesions.1–3 The minor ophthalmic accessory lacrimal glands of Wolfring (tarsi) Krause (fornices) and Popoff (caruncle) may also be considered analogs of the minor salivary glands of the labial, buccal, palatal, and lingual tissues.4,5 This report describes the first corroborated diffuse, strictly parenchymal enlargement of the lacrimal gland, which probably is identical to sialadenosis of the major salivary glands.1 This structure was designated the dacryoadenosis. In the course of thorough histopathologic, immunohistochemical, and electron microscopic investigations of this condition, a distinct population of mucusproducing, morphologically classic goblet cells (mucocytes) was found within many secretory acini. This led to an assessment of the prevalence of such cells in five normal lacrimal glands, in which smaller numbers of goblet cells also were found. During the previous 75 years, the presence of lacrimal gland goblet cells in human tissue has been completely overlooked in anatomic and pathologic studies.4–7 No convincing histopathologic or illustrative evidence has ever been adduced to confirm the existence in the human lacrimal gland of morphologically acceptable goblet cells. ELSEVIER INC. ALL RIGHTS RESERVED. 267 The acinar cells, in addition to synthesizing zymogen secretory granules, recently have been considered to be the putative source of any mucosubstances that the lacrimal gland produces.6,7 This paper provides, for the first time, histochemical and ultrastructural proof that goblet cells indisputably exist in the lacrimal gland. uations in this study were interpreted in the context of comparative data reported in relevant ophthalmic and general pathologic scientific publications. RESULTS SUBJECTS AND METHODS THIS WORK IS A RETROSPECTIVE CLINICOPATHOLOGIC study conducted under a Massachusetts Eye and Ear institutional review board-approved protocol (2019P000339) and in compliance with Health Insurance Portability and Accountability Act regulations and the tenets of the Helsinki Declaration. The patients’ surgical consent form allows publication of nonidentifiable clinical and medical data and appropriately cropped clinical photographs. No additional procedures or tests were performed in the patient beyond the indicated clinical care and diagnostic and treatment regimens. After a review of clinical photographs, medical-records and imaging studies, a case of an excised, dramatically enlarged lacrimal gland with no apparent light microscopic cytologic or inflammatory abnormalities was documented with hematoxylin and eosin (H&E) staining. Additionally, the specimen was intensively evaluated with histochemical and immunohistochemical stains and transmission electron microscopy for the detection of any subtle abnormalities. For electron microscopy, formalin-fixed tissue was deparaffinized and reprocessed with appropriate glutaraldehyde fixation and osmium staining. The histochemical stains included periodic acid–Schiff (PAS), Masson trichrome, Alcian blue, mucicarmine, phosphotungstic acid hematoxylin (PTAH), reticulin silver, and Gomori methenamine silver (for mucus). Immunohistochemical staining included cytokeratins (CK) 7, 14, 19, and 20, smooth muscle actin (SMA), gross cystic disease fluid protein-15 (GCDFP-15), S-100, SOX 10, DOG-1, chromogranin, synapsophysin, and CD 3, CD 20, CD 138, immunoglobulin A (IgA) and IgG. Five microscopically normal lacrimal glands that were biopsied for suspected disease but with negative results served as controls. The medical records of all the patients in this study were carefully reviewed to establish the absence of any diabetic, systemic, endocrine, metabolic, auto-immune or viral diseases that might have had an impact on the cytomorphology or inflammatory cell populations of the evaluated tissues. No evidence of these conditions was found in the patients’ records whose lacrimal glands were selected for inclusion in this study. The selected glands were studied with H&E, PAS, Alcian blue, mucicarmine, Masson trichome, reticulin silver, and Gomori methenamine silver stains. The immunohistochemical stains that were used were CK7, CK14, GCDFP-15 and IgA. The findings from the performed eval268 CLINICAL HISTORY: A 45-year-old white female developed a painless swelling of her lateral left upper eyelid and lateral canthal region that had first appeared 3 years earlier and had slowly become more prominent (Figure 1, upper left). Other symptoms included arthritis, dry eyes, and dry mouth. She had undergone surgery for sinusitis 3 years previously and was receiving latanoprost eye drops for primary open angle glaucoma, fluoxetine for depression, and desloratadine and mometasone for allergies and sinusitis. There was no history or family history of neoplasia. Ocular examination disclosed a prominent S-shaped configuration to her left upper eyelid without skin erythema. There was a firm subcutaneous lacrimal gland. The right gland also felt somewhat enlarged. The palpebral lobe of the left lacrimal gland was prominently visible when the eyelid was retracted and partially everted (Figure 1, top right). Visual acuity, intraocular pressures, fundus examinations, ocular motility evaluation, and visual field results were within normal limits in each eye. Palpation of the head and neck lymph nodes and the parotid and submandibular regions was unremarkable. Magnetic resonance imaging revealed bilateral lacrimal gland enlargement, greater on the left. The glands appeared to be well outlined (Figure 1, middle left and middle right, bottom left and bottom right) with an exaggerated oblong shape protruding beyond the orbital rim and lacking intralesional cysts that might be suggestive of dacryops (Figures middle left and middle right). The entire left lacrimal gland was excised in December of 2008 through an incision in the upper eyelid crease with an extraperiosteal approach. A distinctly enlarged, lobulated, soft, unencapsulated white-pink mass was encountered during surgery. Postoperatively, the patient healed well without ptosis or a motility disturbance. Eleven years after surgery, there was no evidence of a local recurrence nor of any further enlargement of the right lacrimal gland. HISTOPATHOLOGIC FINDINGS IN THE DACRYOADENOTIC GLAND: The gross specimen that was received fresh in the eye pathology laboratory was a single piece of pink and white tissue measuring 26 3 18 3 6 mm. Under lowpower microscopy, lobules of lacrimal tissue displayed varying sizes (Figure 1, bottom left). The largest lobules measured 8-10 mm in greatest diameter and were composed of sheets of acini without any obvious internal septation or a discernible ductular system (Figure 1, bottom right). Most lobules were smaller, in the range of 2.0-2.5 mm in diameter, which may have reflected different levels of tissue sectioning. At intermediate and higher power microscopy, AMERICAN JOURNAL OF OPHTHALMOLOGY MAY 2020 FIGURE 1. Clinicopathologic features of dacryoadenosis. (Top left) A 48-year-old woman developed a painless left upper eyelid and lateral canthal swelling (crossed arrow). The right lacrimal gland was also mildly enlarged (arrow). (Top right) Retraction of the left upper eyelid discloses an enlarged palpebral lobe (arrow) of the lacrimal gland. (Middle left) An axial computed tonogram reveals oblong bilateral enlargements of the lacrimal glands (arrow), greater on the left (crossed arrow). The lesion is contoured to the adjacent bone and sclera. (Middle right) Coronal tomogram displays the larger left gland (crossed arrow) in comparison with the mildly enlarged right gland (uncrossed arrow). (Bottom left) Varying sizes of the lobules of the excised right gland. (Bottom right) A massively enlarged lobule dominates the field. Smaller glandular subunits are also seen (arrows), perhaps at different levels of the enlarged lobules (bottom left and right, H&E stain, original magnification 34 and 312.5). rare interlobular ducts were observed and were sometimes enveloped by collagen (Figure 2, top left). Clusters of ductules with larger lumens occasionally were situated in close proximity to the acinar units (Figure 2, top left, inset). Small periductal lymphoid aggregates were occasionally identified but were fewer in number than usual. The acini were composed of cuboidal to columnar cells that created small lumens and had nuclei positioned in the mid-level of the cytoplasm. They were stuffed with myriad eosinophilic and retractile (zymogen) granules that extended from their apices to their bases (Figure 2, top right). These granules were PAS-positive and diastase resistant. Multiple clustered duct formations emerging from the acini had larger lumens and agranular cytoplasm (Figure 2, upper right). Close inspection of several microscopic fields revealed paler cells without granules VOL. 213 (Figure 2, middle left) that sometimes manifested a delicate, reticulated cytoplasm. Their nuclei were displaced to the basal region (Figure 2, middle right). The cells with pale cytoplasm were accentuated with the Masson trichrome (Figure 2, bottom left) and PTAH (Figure 2, bottom right) stains, which revealed a blue-gray cytoplasmic hue. These stains also demonstrated the presence, respectively, of myriad cytoplasmic red and blue granules in some acinar cells, with absence of granular staining in the pale acinar cells. The Alcian blue stain disclosed that the pale cells were positive for mucus; these cells were either singly dispersed in the acini (Figure 3, top left and top right) or completely replaced them (Figure 3, middle left). The mucicarmine stain also verified the presence of cytoplasmic mucus (Figure 3, middle right) in the pale cells. GOBLET CELLS IN LACRIMAL GLANDS 269 FIGURE 2. Histopathologic features of dacryoadenotic lesion. (Top left) An interlobular duct (D) is surrounded by fibrous tissue with many nearby secretory acini. The inset depicts a cluster of nongranular ductules (D) on the right with granular secretory acini on the left. (Top right) A nongranular trifurcating ductule (arrows) is present in the center of this field amid many granular secretory acini. (Middle left) Pale acini (arrows) are dispersed among granular acini. (Middle right) Higher power photomicrograph of pale staining nongranular acini (arrows). Some of the pale staining cells have a reticulated cytoplasmic appearance. (Bottom left) Masson trichrome stain highlights the pale blue-gray cytoplasm (arrows) that lack red staining granules compared with the surrounding granular acinar cells. (D) Intralobular ductule. (Bottom right) The PTAH (PTAH) stain also demonstrates the absence of blue staining granules in the pale-blue cytoplasm (arrows). (Hematoxylin and eosin stain; top left, original magnification 3200; top right, original magnification 3400; middle left, original magnification 3200; middle right, original magnification 3600; bottom left, original magnification 3600; bottom right original magnification 3400). IMMUNOHISTOCHEMICAL FINDINGS OF THE DACRYOADENOTIC GLAND: Immunostaining for CK7 revealed clus- ters of clear cells in which the cytoplasmic tonofilaments were dislocated peripherally beneath the cells’ limiting membranes (Figure 3, bottom left). GCDFP-15 positivity was strong in the granular acinar cells (Figure 3, bottom right, left panel), negative in the ductules, and mostly effaced in the pale acinar cells due to an absence of cytoplasmic zymogen granules (Figure 3, bottom right, right panel). S-100, CK14, and SMA established a reduction 270 in myoepithelial cells which were only sporadically present at the peripheries of the acini. CK14 but not SMA immunostained the basal cells of the ductules. CK19 was mildly positive in the acini and strongly positive in the ducts. The nuclear stain SOX10 and the luminal membrane stain DOG-1 were both positive; chromogranin and synaptophysin were negative in all cells. CD3, CD20, CD138, and IgG and IgA were detected throughout the stroma, with CD3þ T-lymphocytes outnumbering CD20þ B lymphocytes. IgGþ plasma cells (CD138þ) predominated over IgA- AMERICAN JOURNAL OF OPHTHALMOLOGY MAY 2020 FIGURE 3. Histochemical and immunohistochemical findings in dacryoadenotic lesion. (Top left) The Alcian blue stain shows mucous globules in several acinar cells. (Top right) More diffuse distribution of Alcian blue-positive mucus in the acinar cells. (Middle left) Several adjacent acini are completely replaced by blue staining goblet cells. (Middle right) The mucicarmine test for mucin is magenta-positive but stains the mucus less vividly than the Alcian blue stain. (Bottom left) Cytokeratin 7 immunostaining reveals clearing of the cytoplasm of the acinar cells (arrows) caused by peripheral margination of the cytoplasmic tonofilaments due to the presence of abundant intracellular mucus inclusions in the goblet cells. (Bottom right, left panel) Intense positive cytoplasmic immunoreactivity for GCDFP-15 (GCFGP-15) reflects staining of the acinar cytoplasmic zymogen granules. (Bottom right, right panel) Clearing of the acinar cell cytoplasm (arrows) because of GCFGP-15 negativity due to replacement zymogen granules by goblet cell mucous inclusions. The faint central luminal positivity represents the secretions of zymogenic cells elsewhere in the acinus. (Top left and top right, original magnification 3600; middle left and middle right, original magnification 3600; bottom left and bottom right, original magnification 3400). positive plasma cells. Small scattered periductular lymphocytic aggregates were mostly composed of B-lymphocytes. ULTRASTRUCTURAL FINDINGS OF THE DACRYOADENOTIC GLAND: The acini possessed small central lumens created by surrounding pyramidal cells replete with bounteous electron–dense and electron-lucent cytoplasmic zymogenic granules (Figure 4, top left) that had a mean diameter of 1.3 mm. The electron-dense granules were VOL. 213 overall more numerous than the electron-lucent granules, which had a mean diameter of 1 mm (Figure 4, top right). Outside of the pyramidal cells, along their basal borders, there was an interrupted and incomplete layer of flattened contractile myoepithelial cells (Figure 4, middle left), a finding consistent with the immunohistochemical studies (Figure 4, middle right inset). Many myoepithelial cells had degenerated and disappeared leaving the acinar pyramidal cells resting directly on a basement membrane that GOBLET CELLS IN LACRIMAL GLANDS 271 FIGURE 4. Ultrastructural features of dacryoadenotic lesion. (Top left) Acinar secretory pyramidal cells delimit a small central lumen (L) and contain bounteous electron-dense zymogen granules. (Top right) Some of the secretory zymogen granules are comparatively electron-lucent and are admixed with the dark granules. (Middle left) Contractile myoepithelial cell (MYE) possesses cytoplasmic actin filament (F). This cell is applied to the base of a pyramidal acinar cell. (Middle right) A degenerated myoepithelial cell (MED) has left behind remnants of its actin myofilaments, A pyramidal cell endowed with dark zymogen granules is separated from the stroma only by its basement membrane (white arrows). The acinar cell possesses prominent rough endoplasmic reticulum. The inset discloses the discontinuities in the myoepithelial layer with SMA immunostaining. (Bottom left) A light cell with a subplasmalemmal aggregate of small granules (open white arrows) that represents a heterotopic intracinar terminal ductular cell. Mitochondria (M) and lysosomal type granules (G) are present in the perinuclear cytoplasm. Note the large zymogen-rich adjacent acinar cells. Crossed arrow [ nucleolus; L [ acinar lumen; N [ nucleus. (Bottom right) Electron micrograph of an intracinar goblet cell replete with flocculo-granular mucus inclusions (MG and insert) with the exclusion of the zymogen granules. A few scattered dark granules are either lysosomes or phagocytized melanin granule released from adjacent acinar cells. (Top left, original magnification 32,200; top right, original magnification 32,000; middle left, original magnification 35,600; middle right, original magnification 35,600; bottom left, original magnification 33,400; bottom right, original magnification 32,800; bottom right inset, original magnification 31,800). abutted the stroma. Myofilamentous remnants were sometimes the only trace of the once intact myoepithelial cells (Figure 4, middle right). Sparsely distributed within the acini were scattered heterotopic ductular cells with cyto272 plasmic apical granules that were much smaller (mean diameter of 0.4 mm) than the acinar zymogen granules (Figure 4, bottom left). The mucus-rich goblet acinar cells were swollen with mucous granules that evinced an AMERICAN JOURNAL OF OPHTHALMOLOGY MAY 2020 FIGURE 5. Microscopic and histochemical features of normal lacrimal glands. (Top left) Multiple lacrimal parenchymal lobules are generally smaller than those in the dacryoadenotic lesion. The inset demonstrates the dense granularity of the acinar cells. (Top right) Many of the acinar cells display hypogranularity (arrows) due to discharge of most of their cytoplasmic zymogen granules into the tears. Such relatively clear cells differ from the complete agranularity of the intra-acinar pale staining goblet cells. (Middle left) Pale eosinophilic acinar cells lack zymogen granules intimating the presence of cytoplasmic mucous inclusions. (Middle right) Masson trichrome stain discloses light blue cytoplasm of pale cells (arrows) that lack red staining zymogen granules, which are observed in the adjacent serous acinar cells. (Bottom left) The arrows indicate the presence of Alcian blue-positive mucus material occupying the entire cytoplasm of scattered intra-acinar cells. (Bottom right) Alcian blue positive material sub-totally occupies the supranuclear cytoplasm. (Top left, H&E stain, original magnification 312.5; inset, original magnification 3400; top right and middle left, H&E stain, original magnification 3600; middle right, original magnification 3600; bottom left, original magnification 3600; bottom right, original magnification 3600). amorphous or flocculent, relatively electron-lucent content (Figure 4, bottom right and inset). There were extremely rare zymogen granules in some of the mucusproducing cells. HISTOLOGICAL AND IMMUNOHISTOCHEMICAL STUDIES OF NORMAL LACRIMAL GLANDS COMPARED WITH THE DACRYOADENOTIC LACRIMAL GLAND: Five samples of normal lacrimal glands obtained from biopsies negative for suspected disease were evaluated microscopically for the VOL. 213 detection of goblet cells (mucocytes). The patients in this group were 3 men and 2 women ranging in age from 17 to 62 years old and an average age of 48 years old. The only clinical correlations that were discovered were a mild increase in intralobular fibrosis and an increase in adipocytes in the septa. The individual lobules were generally smaller than those in the dacryoadenosis case, although there were also occasional larger ones (Figure 5, top left). Acinar cells with pale cytoplasm devoid of zymogen granules were dispersed among cells with the usual endowment of granules GOBLET CELLS IN LACRIMAL GLANDS 273 FIGURE 6. Additional histochemical and immunohistochemical features of normal lacrimal glands contrasted with the dacryoadenotic gland. (Top left) Mucicarmine stain reveals the magenta staining of mucus in the acinar goblet cells (arrows). This stain is less effective in disclosing the presence of cytoplasmic mucus in comparison with Alcian blue staining. (Top right) Several adjacent acini are completely composed of mucicarminophilic cells. (Middle left, left panel) The reticulin stain of a normal gland demonstrates a feltwork of inter-acinar stromal fibers separating the lacrimal acini. (Middle left, right panel) There is scant stroma separating the acinar units in the dacryoadenotic lesion. Note that the width of the acini in the normal and abnormal glands is approximately the same, suggesting the absence of individual cellular hypertrophy or hyperplasia. (Middle right, left panel) Positive immunoglobulin A (IgA) immunostaining of normal numbers of inter-acinar stromal plasma cells that are often found in clusters. (Middle right, right panel) In the dacryoadenotic lesion, the population of IgA-positive plasma cells is reduced and widely dispersed. (Bottom left) In both the normal and the dacryoadenotic glands, cytokeratin 7 stains all of the acini and an elongated ductule (arrows). No goblet cells are present in this field. (Bottom right) Main panel on right. In contrast with the dacryoadenotic gland, in the normal lacrimal gland the acini have a continuous outer investment of the secretory pyramidal cells by a layer of flattened myoepithelial cells, disclosed in this panel with cytokeratin 14. Elongated and small panels on the left. In the normal and dacryoadenotic glands the intralobular, interlobular and excretory ducts (D) (shown at low power on top and high power below) manifest cytokeratin 14-positive basal cells. These cells are probably reserve stem cells. They are not myoepithelial cells because they were SMA-negative. (Top left, original magnification 3400; top right, original magnification 3600; middle left, Immunoperoxidase stain, both panels, original magnification 3200 Immunoperoxidase stain; bottom left, original magnification 3400; bottom right panel original magnification 3400; elongated and small bottom panels, original magnification 3200 and 3600, respectively). (Figure 5, top right). Another cell type to be distinguished from the typical acinar cells had a reduction of identifiable granules due to their discharge during the cells’ metabolic 274 cycle; they sometimes had a vaguely reticulated cytoplasm (Figure 5, middle left). The PAS stain highlighted a full complement of zymogen granules in the acinar cells, fewer AMERICAN JOURNAL OF OPHTHALMOLOGY MAY 2020 in the hypogranular acinar cells, and none in the ductular and pale acinar cells. The Masson trichrome stain (Figure 5, middle right) disclosed a similar blue-gray cytoplasmic appearance of the pale cells. Also demonstrated was the absence of red zymogen granules in the ducts and pale cells, which contrasted with the Masson-positive bright eosinophilic-red granules in the normal pyramidal granular cells (Figure 5, middle right). Likewise, the PTAH stain failed to uncover any blue-staining granules. The Alcian blue stain demonstrated a blue mucus content in individual agranular pale cells (Figure 5, bottom left) or in clusters of these cells (Figure 5, bottom right). The mucicarmine and Gomori methenamine silver stains also corroborated the presence of mucus is the pale staining cells (Figure 6, top left and top right). The reticulin stain highlighted a larger number of delicate stromal fibers in the normal glands (Figure 6, middle left, left panel) than in the dacryoadenotic gland (Figure 6, middle left, right panel). Immunostaining for IgA plasma cell was substantially reduced in the inter-acinar stroma of the dacryoadenotic lesion (Figure 6, middle right, left panel) in comparison with their comparative abundance in the normal glands (Figure 6, middle right, right panel). GCDFP-15 was seen in the acinar cells but was absent in the ductules and the pale mucus-positive acinar cells. CK7 was diffusely positive in the granular acinar pyramidal cells and in the intra- and extralobular ductules in both the normal and the dacryoadenotic tissue (Figure 6, bottom left) but undetectable in the pale cells (Figure 3, bottom left). CK14 (Figure 6, bottom right, main panel) and SMA each stained an intact, continuous outer myoepithelial layer around the normal acinar cells but showed discontinuities in the dacryoadenotic lesion. CK14 staining demonstrated positivity in the basal cells of the intralobular and extralobular ductules in normal and lesional ducts (Figure 6, bottom right, elongated and small panels). SMA, however, was negative within the basal cells in both the normal and the dacryoadenotic glands but strongly positive in the intact myoepithelial layer of the normal glands along with CK14 (Figure 6, bottom right, main panel on right). This finding contrasted with the discontinuities in the myoepithelial layers in the dacryoadenotic gland. DISCUSSION THE NEW LACRIMAL GLAND LESION DESCRIBED IN THIS report is unlike any other epithelial disorders of this structure that has been previously reported.2 It is therefore essential to provide some background information and an overall review of lacrimal gland structure and functioning to grasp its distinctive and exceptional nature. MICROANATOMY AND BASIC PHYSIOLOGY OF THE LACRIMAL GLAND: To understand the fundamental path- VOL. 213 ologic alterations of the lacrimal and salivary glands in dacryoadenosis and sialadenosis, one must be familiar with the obvious and subtle normal features of these analogous but not totally identical structures.4,5,8,9 The main lacrimal gland and the accessory lacrimal glands, like the parotid gland and other salivary glands, have a tubaloracemose (alternatively called tubuloacinar) architecture. Grossly, the lacrimal gland is an almond-shaped and unencapsulated structure directly abutting the orbital fat and located in the anterolateral superior orbit. The lateral horn of the levator aponeurosis divides the gland into a larger posterior orbital lobe (two-thirds of the total size) and a smaller superficial anterior palpebral lobe (onethird). The lobules in the palpebral lobe are smaller than those in the orbital lobe. Ten to fifteen excretory ducts, many originating in the deep orbital lobe, pass through the palpebral lobe to deliver tears to the superolateral conjunctival sac. Hence, total excision of the palpebral lobe results in ablation of tear flow from a major gland; however, the accessory glands of Krause (conjunctival fornix), Wolfring (tarsus), and Popoff (caruncle) continue to secrete a baseline level of tears. Microscopically, the lacrimal gland is constituted by lobules that are separated from each other by fibrous septa that sometimes contain adipocytes. The lacrimal lobules comprise of collections of acini with small lumens generally believed to contain only serous granular cells,4,5 whereas the parotid gland is composed of seromucinous acini containing an admixture of goblet cells.9 The serous cells produce zymogen granules. Throughout this discussion the terms serous, granular, and zymogen-producing have been used interchangeably. There are intralobular and interlobular collecting ducts without zymogen granules that converge to create the terminal excretory ducts that contain scattered goblet cells.4,6,10,11 In addition to the serous cells of the parotid and lacrimal glands, zymogen granules are also found in the chief cells of the gastric mucosa, the Paneth cells of the small intestine, and the acinar cells of the exocrine pancreas.9 It has been a matter of dogma that the lacrimal acini lack a separate population of mucus-producing goblet cells (mucocytes), despite the gland’s known ability to synthesize mucosubstances.6,7,11,12 The latter have been regarded as another secondary product of the acinar cells that primarily synthesize zymogen granules. Like the parotid gland, the accessory salivary glands (labial and buccal) are also composed of seromucinous cells. The glands in the lingual circumvallate papillae, however, are completely serous; on the other hand, the palatal glands are entirely mucinous.9 The accessory lacrimal glands are currently regarded as entirely serous, like the major lacrimal glands. The secretory units of the lacrimal gland are composed of pyramidal cells that form an inconspicuous central lumen. A monolayer of continuous flattened, contractile myoepithelial cells buttresses the outer aspect of the acini and facilitates expulsion of the tears.2,4,5 Of interest is the fact GOBLET CELLS IN LACRIMAL GLANDS 275 that myoepithelial cells are also found in mammary, prostate, eccrine, and apocrine glands.9 Electron microscopy discloses that the pyramidal cells possess abundant profiles of rough surfaced endofilament reticulum that synthesize large electron-dense and electron-lucent zymogen granules measuring 0.5 to 1.4 mm in diameter and that are preferentially located in the apical region of the cells.4,5,8 Golgi lamellae for product packaging are concentrated in the mid-level and the basal cytoplasm. Zymogen granules are compact collections of inactive pro-enzymes that require hydrolysis by a cofactor to be activated. In this regard, they differ from others secretory granules. Zymogen granules containing different pro-enzymes are also found in the pancreas, gastric and intestinal mucosae, and parotid gland. The light-to-dark ratio of lacrimal zymogen granules is variable from cell to cell, and differences in their exact contents await further elucidation. The intralobular ducts can be distinguished from acinar cells by their larger lumens and the absence of cytoplasmic zymogen granules which can be highlighted by the PAS stain. The ductular cells exhibit smaller granules measuring around 0.4 mm in diameter8; the large excretory ducts lack granules but have scattered mucocytes.6,7,10 Stimulation of the mostly aqueous lacrimal secretion is supplied by autonomic parasympathetic post-ganglionic nerve radicles, while the sympathetic terminal nerves influence tear production through regulation of vascular flow mediated by myoid mural cell contractions.13 A delicate interacinar collagenous interstitium contains myriad plasma cells (estimated to be 3 million in each gland)14 that contribute IgA to the tears. T cells and IgG-positive plasma cells are also present in the stroma. B lymphocytes are fewer in number and usually located in the periductular regions in small primary follicles.15 The B-cell aggregates may represent a reserve population for the recruitment of IgG and IgA interacinar plasma cells. These indigenous lymphocytic cells of the gland often mislead general pathologists unfamiliar with the gland’s normal structure into the trap of diagnosing dacryoadenitis. This diagnosis requires prominent lymphoid aggregates, often with follicular organization; an increased presence of interacinar mononuclear inflammatory cells; progressive interacinar and periductal fibrosis; focal, segmental or diffuse acinar destruction; and, finally, the preferential survival of the preacinar ductules. The normal lining epithelium of the intralobular and interlobular ducts does not display goblet cells, which do appear in the more distal segments of the excretory ducts6,7 along with small pseudoapocrine apical snouts.10 The excretory ducts are formed by the confluence of the interlobular ducts and empty into the superolateral conjunctival fornix. The excretory ducts may therefore be expected to retain some of the features of the adult conjunctival epithelium.4 Ultrastructurally, the cells lining the lacrimal ducts display collections of small apical granules whose contents are presumably different from those of the larger acinar zymogen granules.8 276 All of the intralobular and extralobular excretory ducts manifest a basal epithelial layer that does not show evidence of myoepithelial differentiation and probably represents a germinal cell type.4,8 Germinal cells are not found in the acini, which are composed of cells in a terminal differentiation state (post-mitotic) serous-granular pyramidal cells and myoepithelial cells. The acini consequently cannot be replaced if destroyed by inflammation. Despite its structural simplicity and monotonous architecture, the lacrimal gland has been determined to synthesize and secrete a wide variety of biologically active products. Some of these molecules may be constituents of the acinar zymogen granules. Thirty years ago, the main proteins identified in the tears were lactoferrin and lysozyme.12 One of the more interesting recent discoveries of lacrimal protein synthesis has been the identification of lacritin.16–25 This moiety of acinar cell secretions (which is also found in breast tissue) appears to sustain baseline tear production, maintains cellular homeostasis, promotes corneal surface integration, reduces autophagy, and plays a role in the corneal neuropathy of Sjogren’s syndrome, in which its tear levels are reduced, thereby exacerbating the disease. Over the years, a remarkable number of other secreted molecules has been documented.12 Antibacterial factors consist of phospholipase A2, lysozyme, peroxidase, tear specific pre-albumin, and around a half-dozen different mucins. Also contributed to the tears are retinol and growth factors such as epidermal, fibroblastic, hepatocytic, keratinocytic, and transforming growth factor beta. In human lacrimal glands, mucins are contributed by acinar and excretory duct goblet cells, which incidentally make up all of the cells of the rabbit duct system.11 ADENOSIS OF THE LACRIMAL AND SALIVARY GLANDS: Sialadenosis and its close if not identical relative dacryoadenosis are constituted by increased numbers of cells and acini that result in the glands’ overall enlargement.1 The parotid gland condition, the most common site that is affected, is typically bilateral but rarely may be unilateral. The disease causes a decrease in the production of saliva and a dry mouth ensues; it usually becomes clinically apparent in the fifth and sixth decades with a slight female predominance. A systemic disorder is associated with the salivary gland enlargement in most cases, for example, diabetes mellitus, chronic alcoholism, anorexia nervosa and bulimia, and malnutrition.1,26 Recently the ingestion of certain drugs has been incriminated in a subset of cases, such as valproic acid for epilepsy27 and catecholamine (epinephrine) inhalants for bronchodilation.28 According to current knowledge, a minority of cases are idiopathic, lacking any known underlying disease.1 The reigning theory about the common causation of sialadenosis is that it is due to a neuropathy of the parasympathetic arm of the autonomic nervous system.29 The parasympathetic supply to the lacrimal glands travels for a short distance within the facial nerve and then branches off to join the Vidian AMERICAN JOURNAL OF OPHTHALMOLOGY MAY 2020 nerve, which synapses in the pterygopalatine ganglion.5 The post-ganglionic nerves then are grouped into the lacrimal nerve that stimulates tear production.13 Any pathologic impingement or damage inflicted along this extended neural route could lead to dysfunction or denervation of the parasympathetically generated signals to the acini, causing failure to discharge their zymogen granules which consequently accumulate and mechanically bloat the individual acinar cells. The patient develops a dry mouth and dry eye. ANALYSIS OF THE CURRENT DACRYOADENOTIC LESION: The present case of lacrimal gland enlargement developed in a 45-year-old female who presented with mild left superolateral eyelid and lateral canthal swelling of approximately 3 years’ duration. She also complained of dry eyes and a dry mouth and had previously undergone surgery for sinusitis. The eye and oral dryness implied concurrent decreased tear and saliva secretion, respectively. It should be pointed out that dacryoadenosis may be undetected because the lacrimal gland is rarely if ever palpated, nor is the upper eyelid everted often enough to permit examination of the palpebral lobe beneath the conjunctiva. Eversion of the current patient’s upper eyelid disclosed a prominent palpebral lobe. This failure to evert the eyelid is especially true for head and neck specialists but, unfortunately, is also too frequent a lapse on the part of many ophthalmologists. This neglect may therefore account for the previous failure to recognize the possibility of concurrent parotid-lacrimal or isolated lacrimal adenotic disease. The overhang of the orbital boney rim may further impede detection of moderate degrees of gland enlargement. There was no specific comment in the current patient’s medical record regarding the state of her parotid glands: however, palpation of the head and neck lymph nodes was unrevealing, which presumably would have included the parotid glands during the examination. Magnetic resonance imaging demonstrated bilateral, diffuse, oblong lacrimal gland enlargements, greater on the left, with smooth contours. The larger of the 2 glands measured 26 3 18 3 6 mm; magnetic resonance images of normal glands have exhibited average measurements of 20 3 12 3 5 mm.30 The bilateral glands’ anteroposterior oblong shape suggested involvement of both the palpebral and the orbital lobes.31 There was an absence of bone changes, including no sign of an accentuation of the normal lacrimal osseous fossa. In contrast, epithelial tumors of the gland create round or ovoid shapes that usually arise from the deep and larger orbital lobe and overwhelmingly spare the palpebral lobe. The deep orbital lobe tumors are frequently associated with contiguous osseous alterations, either accentuating the lacrimal fossa in benign tumors, or producing frank osseous destruction in malignant tumors.31 A complete excision of the left lacrimal gland through the upper eyelid was performed for diagnostic and cosmetic reasons. The right lacrimal gland enlargement was not bioVOL. 213 psied because it was felt to represent the same process as that on the left and had caused only minor eyelid fullness and disfigurement. Grossly, the totally excised lacrimal gland was obviously enlarged. The greatest enlargement was in the anteroposterior dimension due to a limitation of other directional expansions imposed by the lateral boney orbital wall and the firm unyielding sclera. Histopathologically, the acini in the present case of dacryoadenosis, like those in sialadenosis, were tightly arranged and composed of highly eosinophilic cells owing to constipation with myriad granules that were clearly visible in H&E and PAS stained sections. Overall there appeared to be many more acini than usual, thereby creating larger lobules. The accumulation of zymogen granules suggested cell hypertrophy or even hyperplasia, but these impressions were actually illusory, caused by the secondary factor of cytoplasmic engorgement with zymogen granules. The granules were observed microscopically and ultrastructurally to be located both apically and basally in the pyramidal acinar cells. As in sialadenosis1 the outer myoepithelial cellular layer was focally attenuated, degenerated or totally absent, features confirmed with SMA immunohistochemical staining and electron microscopic examination (additional ultrastructural and immunohistochemical results are analyzed below). In the regions lacking myoepithelial cells, basement membrane material adhered to the basal plasmalemmas of the pyramidal cells and insulated them from the interstitial stroma. Sometimes there were only myofilamentous remnants of degenerated myoepithelial cells near the base of the acinar cells. Another lacrimal gland disorder that has been described in Goldenhar syndrome32 displays a semi-selective loss of lacrimal acinar and myoepithelial cells caused by an overgrowth and replacement of the acini by preacinar, terminal periductular squamous (presumably germinal) cells (CK5/6, CK14, and P63-positive but SMA-negative). These squamoid germinal cells were sometimes admixed with rare surviving myoepithelial and pyramidal acinar cells. Many lobules of the involved gland, however, were unpredictably spared. In the current case of dacryoadenosis, the intralobular ducts were sparse and difficult to find. Occasional backto-back clusters of ducts were identified or malformations were observed such as ductular trifurcations. These irregular ductular features may partially explain the low level of tear secretion along with any parasympathetic autonomic dysfunction29 and may explain why the acinar cells were stuffed with nondischarged granules, in other words, there was a blockage of the egress of the acinar granules into the tears because of a reduction in the number of accessible ducts. The scant interstitial stroma was composed of wispy collagen fibers with a reduction of mononuclear inflammatory cells. Juxtaposed fields stained immunohistochemically for IgG-A prepared from the dacryoadenosis lesion compared to normal glands showed fewer such cells in dacryoadenosis. Small lymphoid aggregates without follicular organization were occasionally GOBLET CELLS IN LACRIMAL GLANDS 277 present periductally or near blood vessels, but again they were less conspicuous than in normal lacrimal glands. Reticulin silver stain, which has an affinity for delicate protocollagen stromal filaments and basement membranes, was highly revealing and deserves particular attention. In comparison with the dacryoadenosis tissue, the normal lacrimal tissue had more interacinar reticulin fibers, that is, the acini in dacryoadenosis were more closely packed in comparison with those in normal glands. More insightful, however, was the discovery that the width of the individual acinar units revealed by their reticulin outlines in dacryoadenosis was no larger than those of normal acini. This finding provides additional evidence in support of the absence of true hyperplasia or even hypertrophy of the acinar cells in dacryoadenosis. The overall glandular enlargement was consequently created by an increased number of acini. Thus, the cytoarchitectural basis for the dacryoadenosis is hyperplasia of the number of acini composed of normal-sized cells, rather than an increased number of cells per acinus. IDENTIFICATION OF GOBLET CELLS (MUCOCYTES) IN THE DACRYOADENOTIC LACRIMAL GLAND: The most startling feature in the current case of dacryoadenosis was the discovery of goblet cells in scattered acini, either partially or completely or subtotally replacing the serous/ zymogen cells. A review of standard textbooks and recently published review articles on lacrimal gland structure and function have failed to provide any conclusive proof establishing the presence of lacrimal gland mucocytes.4–8 In H&E-stained sections the goblet cells were noneosinophilic and displayed a faint blue-gray cytoplasmic hue devoid of any stainable zymogen granules. The nuclei were displaced to a basal location by the cytoplasmic mucus. Both the Masson trichrome and PTAH stains highlighted the pale cytoplasm of the mucusproducing cells and the absence of cytoplasmic zymogen granules, contrasting with the vividly Masson trichromepositive red granules or the PTAH-positive blue granules of the adjacent acinar serous/granular cells. A paper published in 197233 reported the existence of mucosubstance in some lacrimal gland cells (large type A), but tissue culture results pointed to smaller serous cells (referred to as type B) as the site for sialic acid mucosubstance localization. Several reviews imply or state that the presence of goblet cells in the lacrimal gland has not yet been convincingly confirmed.6,7,12,34,35 A study of rabbit lacrimal glands demonstrated acinar cells with combined mucinous and serous features.11 The magnification of the published illustrations was too low to ascertain whether any of the acinar cells staining positively with Alcian blue and mucicarmine displayed the morphology of true goblet cells. In one major study of the human lacrimal system6 a handful of anchoring mucins (MUC1, 4, and 16) and secretory mucins (MUC5B and MUC7) were detected and shown to be associated with the acinar cells. MUC subtypes were not observed in the inter-and 278 intralobular ducts, but MUC 5AC was found in the goblet cells of the large terminal excretory ducts.6,35 In 2 papers, it was explicitly stated that the mucinous material is localized to the acinar cells.34,35 According to the foregoing research findings, the investigators proposed that the main mucinpositive cells are acinar pyramidal cells, presumably possessing a dual capacity to synthesize mucin and zymogen granules as well as the other proteins or glycoprotein products found in the zymogen granules and tears. These findings are in a sense analogous to the characteristics of the conjunctival epithelium, in which the tonofilament–rich keratinocytes also synthesize small packets or vesicles of mucosubstance,36 whereas the conjunctival goblet cells are responsible for most of the mucus production.37 A recent investigation reinforces the parallelism between lacrimal acinar cells and conjunctival squamous cells, both of which evince non-goblet cell types that produce similar mucins in tandem.37 In H&E-stained sections, the acinar cells in normal human lacrimal glands have been noted for some time to have variable degrees of cytoplasmic granularity and intensities of eosinophilia, which has been interpreted to represent different levels of discharge of the zymogen granules at different stages of their metabolic cycle. Such relatively ‘‘clear’’ or degranulated cells should not be confused with the authentic pale goblet cells. Although the trichrome and PTAH stains accentuated the clear or gray cytoplasm of the goblet cells in dacryoadenosis, they also revealed a miniscule dispersion of cytoplasmic granules, many of which may be admixed mitochondria. Scant cytoplasmic small granules accompanied by more numerous mitochondria were also detected in the goblet cells with transmission electron microscopy. The scenario that most likely explains these mucocytic granules is that they represent those discharged from adjacent bloated acinar cells into the intercellular space, wherefrom they were taken up secondarily through phagocytosis by the goblet cells. ULTRASTRUCTURAL FEATURES OF DACRYOADENOSIS: The electron microscopic studies disclosed remarkably well-preserved subcellular details of the deparaffinized tissue from the dacryoadenosis lesion, especially the full extent of the serous acinar cells’ endowment with bounteous large electron-dense and electron-lucent secretory granules; no macrogranules, however, were observed. Goblet cells that were engorged with amorphous or flocculent cytoplasmic mucous inclusions resembling those in the goblet cells of the conjunctiva4 were also sporadically seen in some acini and occasionally formed clusters that completely replaced the pyramidal cells. A curiosity seen in the ultrastructural photomicrographs was the rare presence in some acini of scattered heterotopic ductular cells. The latter contained the usual cytoplasmic organelles but also possessed small apical, subplasmalemmal granules distinctively found in normal adlumenal ductular cells,8 which contrast with the large zymogen granules of the AMERICAN JOURNAL OF OPHTHALMOLOGY MAY 2020 acinar cells. These duct-like intra-acinar cells may be interpreted as evidence of the multipotentiality of the acinar pyramidal cells for a double-differentiation or even for metaplasia into duct cells. Alternatively, they may simply represent a displacement of preacinar terminal ductular cells (a transition zone) into an acinus. The adlumenal, subplasmalemmal small, dense, core granules in the heterotopic cells could also be considered, based on morphologic grounds, presumptive evidence of neurosecretory differentiation. Support for a neuroendocrine cellular lineage was sought with immunohistochemical cytologic staining for S-100, chromogranin, and synaptophysin, all of which were negative. On the other hand, both SOX10 and DOG-1 stains were positive, lending some superficial support for the fundamental functional integrity of the dacryoadenotic acinar units. GOBLET CELLS IN NORMAL LACRIMAL GLANDS: The discovery of acinar goblet cells in the current case of dacryoadenosis prompted a search for these cells in a group of 5 normal lacrimal glands. These control glands were obtained from 3 males and 2 females who ranged in age from 17-62 years old and had negative lacrimal gland biopsies to rule out a suspected disease (eg, sarcoidosis or idiopathic dacryoadenitis). The main findings associated with progressive aging noted in this group of glands were a mild to moderate increase in the amount of interacinar fibrotic stroma and an increase in interlobular septal adipocytes, both with advancing age. The population of stromal lymphocytes and plasma cells was not increased with aging, nor was there dilation of the ductular system, a feature that has been reported before in a larger series.38 The normal lacrimal gland tissues were stained with H&E, PAS, Alcian blue, mucicarmine, reticulin, Masson trichome, and PTAH and evaluated at 3 levels of sectioning. A handful of immunohistochemical stains was used, the same used to evaluate the dacryoadenosis lesion. In all 5 specimens, with careful microscopic examination informed by experience derived from the dacryoadenotic lesion, clear, nongranular cells (PAS- and PTAHnegative) were found in H&E-stained sections. These cells were highlighted with the Masson trichrome stain that showed them to have gray-blue cytoplasm. They were dispersed individually, focally and sparsely, or else in a pattern that partially or totally replaced the serous cells of an entire acinus. The Alcian blue and mucicarmine stains unequivocally disclosed that these clear cells harbored abundant mucus in the morphologic form of goblet cells (mucocytes). The latter accounted for approximately two percent of the acinar cells. This control group of lacrimal glands compellingly establishes that mucus cells lurk within lacrimal acini on a normal anatomic basis but require meticulous scrutiny and special stains for their convincing detection. The authors seriously doubt that these cells represent exhausted serous cells depleted of their discharged zymogen granules during tear secretions; nor is VOL. 213 it likely that one differentiated cell type (acinar serous cell) would undergo metaplasia into another fully differentiated cell type (goblet cell). A relevant observation is that highly differentiated cells like the ciliated columnar respiratory epithelium may undergo metaplasia into a simpler squamous epithelium, whereas the reverse is virtually never seen. In dacryoadenosis there is an exaggerated population of an already established population of mucocytes, which can account for up to 5 percent of the acinar cells. SUPPLEMENTAL IMMUNOHISTOCHEMICAL FINDINGS: Besides demonstrating a selective loss of myoepithelial cells in dacryoadenosis mentioned above, immunohistochemistry contributed other valuable ancillary information. The 2 most useful cytokeratins were CK7 and CK14. In common with normal lacrimal glands, CK7 immunostaining of the dacryoadenosis lesion was positive in the lachrymal zymogenic acinar (pyramidal) cells and the adlumenal cells of the ductular system, but not in the myoepithelial cells. CK7 further demonstrated the clear cytoplasm of the goblet cells in which the mucous inclusions had crowded out the cytoplasmic CK7-positive tonofilaments so that they were displaced to the periphery adjacent to the cell membrane. CK 14 stained the myoepithelial cells and ductular basal cells. CK20 was totally negative in all the parenchymal and ductular cells. The clear cell phenomenon also displayed weak GCDPF-15 immunostaining, which highlighted residual granules in the lumen and cytoplasm of the acinar pyramidal cells but did not stain the cytoplasmic mucous inclusions of the goblet cells. The duct system was negative for this biomarker because duct cells do not synthesize zymogen granules. CK14 staining of the myoepithelial cells coexisted with SMA and S-100 positivity. SMA was negative in the basal ductular cells, establishing that they were not myoepithelial but rather germinal cells. It is intriguing that the ectodermally derived myoepithelial cells of the acini co-express both SMA and cytokeratin. Ultrastructural evaluation in the dacryoadenosis lesion failed to disclose a dual filamentary system. Cytokeratin filaments are wider than the actin myofilaments, so that the latter may be obscured. However, actin can also be present in a soluble form in the cytoplasm of the myoepithelial cell as in other cell types (eg, endothelial cells39). Alternative immunohistochemical stains that are helpful in identifying myoepithelial cells are desmin, caldesmon, p63, and glial fibrillary acidic protein. Vimentin can be coexpressed with cytokeratin in neoplastic myoepithelial cells; these cells are found in many salivary/lacrimal tumors, such as pleomorphic adenomas and to a lesser extent in adenoid cystic carcinomas. In fact, there are rare monophasic myoepithial benign and malignant tumors that have been well characterized in the lacrimal glands.2 PUTATIVE ORIGIN OF LACRIMAL GOBLET CELLS: As an out-growth into the anterolateral orbit from the embryonic forniceal conjunctival epithelium, the major lacrimal GOBLET CELLS IN LACRIMAL GLANDS 279 anlage probably retains the capacity to initially differentiate, or less likely undergo metaplasia, into mucusproducing goblet cells. There is a report of a lobule of lacrimal glandular tissue (a dacryoadenoma) originating in the lower epibulbar conjunctival epithelium that was either an acquired or a congenital lesion40 and was separate from the glands of Krause. This lesion contained a generous admixture of goblet cells. A metaplastic phenomenon in the lacrimal tissue may be triggered by the systemic diseases associated with sialadenosis,1 but the current patient was not known to have suffered from any of these conditions. A more straightforward and favorable proposition is that lacrimal goblet cells are intrinsic to the gland’s makeup and are normally present from birth onwards. A recent intriguing and possibly relevant finding concerning lacrimal mucus cells is represented by 5 cases of a new and exceptional parotid neoplasm designated mucoacinar carcinoma that combines features of a mucoepidermoid carcinoma with those of an acinic cell carcinoma.41 In this tumor granular cells were admixed with mucus cells. This entity has implications for the potentiality of salivary and possibly lacrimal acinar parenchymal cells for dual cellular phenotypes emerging during neoplastic transformation. Alternatively there might be an origin from multipotential terminal (preacinar) basal ductular cells.42 The transitional zone where ductules emerge from the acini probably harbors a population of reserve stem cells that are in all likelihood metastable, plastic and mutable due to their intact mitotic capability.2,8,43 Such cells are cognate with those in the corneoscleral limbal epithelium and at the interface between the endocervix/exocervix and esophageal/gastric mucosae. These germinal cells are visibly proliferative in idiopathic dacryoadenitis at its scarifying end stage, at which a plethora of ductules follow atrophy of the acini. Such transition-zone stem cells may well account for several lacrimal gland tumors including pleomorphic adenoma, adenoid cystic carcinoma, and benign and malignant myoepithelial tumors.2,8,42 It is within the realm of possibility that these stem cells possess the ability to produce hybrid or dual cellular phenotypes–an example being the acinar cells and their sibling goblet cells. SOX10 has been documented to play a role in the differentiation of stem cells (the basal ductular cells next to the acini) into full-fledged acinar secretory cells.43 The presence of ductular cells in some dacryoadenotic acini lends credence to this hypothesis, as well as the invasion of acini by preacinar duct cells in the lacrimal gland in Goldenhar’s syndrome.32 Presently, validation of the metaplastic theory of the origin of goblet cells within normal and abnormal lacrimal acini is questionable. Furthermore, as mentioned above, metaplasia generally proceeds from more differentiated cells into less differentiated ones, rather than from one differentiated phenotype to another differentiated phenotype. A more supportable proposition based on the present group’s findings is that the goblet cells in the lacrimal parenchyma 280 are an intrinsic anatomic endowment from birth. Further pathologic and experimental investigations will be necessary for a definitive resolution of this matter. DIFFERENTIAL DIAGNOSIS OF DACRYOADENOSIS: The differential diagnosis of dacryoadenosis includes conditions that cause diffuse, oblong enlargements of the lacrimal gland, which indicate involvement of both the palpebral and the orbital lobes. These are composed mostly of inflammatory conditions that extend from idiopathic dacryoadenitis (a component of the spectrum of idiopathic orbital inflammations, formerly called ‘‘pseudotumors’’), lymphoid tumors, sarcoidosis, Sjogren’s syndrome, vasculitis, and IgG-4-related disease.31 Ingestion of certain drugs can result in a diffuse, noninflammatory expansion of the parotid gland1; they may eventually be found to be responsible for cryptic cases of dacryoadenosis. The latter, however, may be difficult to appreciate due to the overhang of the orbital bony rim and the all too infrequent palpation of the lacrimal gland region or eversion of the upper eyelid. To date, drugs responsible for sialadenosis have been antifertility agents, valproic acid for epilepsy,27 drugs for treating systemic hypertension, and lately, epinephrine used for controlling asthma.1,27,28 Withdrawal of the drugs can reverse the swelling of the parotid gland. Resolution, on the other hand, does not happen when there is a successful treatment of the systemic disease (eg, hypertension, anorexia nervosa, bulimia, malnutrition, alcoholism, and others) associated with sialadenosis. A conceptually rather confusing differential diagnostic entity is adenomatoid hyperplasia.44–46 This benign and rare disorder occurs preferentially in men 30-60 years old rather than in women. Instead of a diffuse process involving the entirety of the gland, adenomatoid hyperplasia represents a focal nodular lesion constituted by normal, hyperplastic parenchymal glandular tissue composed of acini and ducts. The nodules are uninflamed and encircled by a fibrous pseudocapsule. The nodules may be somewhat larger than normal lobules. Most cases arise in the palate, where the accessory salivary tissue is entirely mucinous, as are the nodules. The parotid and submandibular glands are characteristically spared. When the small accessory oral glands (lingual, buccal, labial) are rarely involved, there can be difficulty in appreciating the nodule due to the small size of the pre-existent normal accessory gland in which the lesion arises. The lacrimal gland has not yet been reported to generate this kind of lesion, probably because it is predominantly a nonmucinous serous gland. No specific cause for this lesion has been found, that is, none of the systemic diseases associated with sialadenosis are detected. Simple excision is performed without recurrence and the clinical course is uneventful. TREATMENT OF SIALADENOSIS AND DACRYOADENOSIS: The most common surgical treatment for persistent and refractory parotid sialadenosis is subtotal excision of the swelling.1 Oral pilocarpine has allegedly induced some AMERICAN JOURNAL OF OPHTHALMOLOGY MAY 2020 reduction in the swelling in a few patients with bulimia. For patients whose parotid and sublingual swellings are left alone there is progressive involution of the excessive parenchymal tissue. Despite this event, the glands remain enlarged because of replacement by the adipose tissue that is in amounts equal to the involuted glandular tissue. In the current dacryoadenosis case total excision of the larger gland was curative. The contralateral lacrimal lesion that was excised has not undergone further enlargement from the clinical vantage point after eleven years of follow up. CRediT AUTHORSHIP CONTRIBUTION STATEMENT FREDERICK A. JAKOBIEC: CONCEPTUALIZATION, METHOD- ology, Writing - original draft, Writing - review & editing. Ralph C. Eagle: Writing - original draft, Writing - review & editing. Martin Selig: Methodology. Lina Ma: Writing original draft, Data curation. Carol Shields: Writing - review & editing. ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST and none were reported. Funding/Support: Supported by the Massachusetts Eye and Ear Department of Ophthalmology discretionary research fund. Financial Disclosures: The authors have reported that they have no relationships relevant to the contents of this paper to disclose. REFERENCES 1. Ellis G, Auclair P. Tumors of the salivary glands. Washington, DC: American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology; 2008:506–514. 2. Grossniklaus H, Eberhart C, Kivelä T. Tumours of the lacrimal glands WHO classification of tumours of the eye. 4th ed. Lyons: International Agency for Research on Cancer; 2018:150–165. 3. El-Naggar A, Chan J, Grandis J, Taketa T, Slootweg P, eds. WHO Classification of Head and Neck Tumours. 4th ed. Lyons: International Agency for Research on Cancer; 2017:159–202. 4. Jakobiec F, Iwamoto T. The ocular adnexa. In: Fine B, Yanoff M, eds. Ocular Histology: An Atlas and Text. 2nd ed. Hagerstown, MD: Harper & Row; 1979:289–342. 5. Bron A, Tripathi R, Tripathi B. Wolff’s anatomy of the eye and orbit. 8th ed. London: Chapman & Hall Medical; 1997:72–73. 76, 194. 6. Paulsen F. Cell and molecular biology of human lacrimal gland and nasolacrimal duct mucins. Int Rev Cytol 2006; 249:229–279. 7. Paulsen F, Berry M. Mucins and TFF peptides of the tear film and lacrimal apparatus. Prog Histochem Cytochem 2006;41: 1–53. 8. Iwamoto T, Jakobiec F. A comparative ultrastructural study of the normal lacrimal gland and its epithelial tumors. Hum Pathol 1982;13:236–262. 9. Ross M, Pawlina W. Histology: a text and atlas. 6th ed. Philadelphia: Wolters Kluwer, Lippincott Williams and Wilkins; 2011:527. 546–551, 578–579, 593, 646–647. 10. Jakobiec FA, Zakka FR, Perry LP. The cytologic composition of dacryops: an immunohistochemical investigation of 15 lesions compared to the normal lacrimal gland. Am J Ophthalmol 2013;155:380–396. 11. Ding C, Huang J, MacVeigh-Aloni M, Lu M. Not all lacrimal epithelial cells are created equal-heterogeneity of the rabbit lacrimal gland and differential secretion. Curr Eye Res 2011;36:971–978. VOL. 213 12. Conrady C, Joos Z, Patel B. Review: the lacrimal gland and its role in dry eye. J Ophthalmol 2016;7542929. 13. Dartt D. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res 2009;28:155–177. 14. Allansmith M, Kajiyama G, Abelson M, Simon M. Plasma cell content of main and accessory lacrimal glands and conjunctiva. Am J Ophthalmol 1976;82:819–826. 15. Wieczorek R, Jakobiec F, Sacks E, Knowles D. The immunoarchitecture of the normal human lacrimal gland. Relevancy for understanding pathologic conditions. Ophthalmology 1988;95:100–109. 16. Feng M, Baryla J, Liu H, et al. Cytoprotective effect of lacritin on human corneal epithelial cells exposed to benzalkonium chloride in vitro. Curr Eye Res 2014;39:604–610. 17. Fujii A, Morimoto-Tochigi A, Walkup R, Shearer T, Azuma M. Lacritin-induced secretion of tear proteins from cultured monkey lacrimal acinar cells. Invest Ophthalmol Vis Sci 2013;54:2533–2540. 18. Karnati R, Talla V, Peterson K, Laurie G. Lacritin and other autophagy associated proteins in ocular surface health. Exp Eye Res 2016;144:4–13. 19. McKown R, Wang N, Raab R, et al. Lacritin and other new proteins of the lacrimal functional unit. Exp Eye Res 2009; 88:848–858. 20. McNamara N, Ge S, Lee S, et al. Reduced levels of tear lacritin are associated wIth corneal neuropathy in patients with the ocular component of Sjögren’s syndrome. Invest Ophthalmol Vis Sci 2016;57:5237–5243. 21. Wang N, Zimmerman K, Raab R, et al. Lacritin rescues stressed epithelia via rapid forkhead box O3 (FOX03)-associated autophagy that restores metabolism. J Biol Chem 2013; 288(25):18146–18161. 22. Samudre S, Lattanzio F Jr, Lossen V, et al. Lacritin, a novel human tear glycoprotein, promotes sustained basal tearing and is well tolerated. Invest Ophthalmol Vis Sci 2011;52: 6265–6270. 23. Sanghi S, Kumar R, Lumsden A, et al. cDNA and genomic cloning of lacritin, a novel secretion enhancing factor from the human lacrimal gland. J Mol Biol 2001;310:127–139. GOBLET CELLS IN LACRIMAL GLANDS 281 24. Ubels J, Gipson I, Spurr-Michaud S, Tisdale A, Van Dyken R, Hatton M. Gene expression in human accessory lacrimal glands of Wolfring. Invest Ophthalmol Vis Sci 2012;53: 6738–6747. 25. Weigelt B, Bosma A, van’t Veer L. Expression of a novel lacrimal gland gene lacritin in human breast tissues. J Cancer Res Clin Oncol 2003;129:735–736. 26. Garcia Garcia B, Dean Ferrer A, Diaz Jimenez N, Alamillos Granados F. Bilateral parotid sialadenosis associated with long-standing bulimia: a case report and literature review. J Maxillofac Oral Surg 2018;17:117–121. 27. Derin H, Derin S, Oltulu P, Özbek O, Çaksen H. Pediatric sialadenosis due to valproic acid. J Craniofac Surg 2017;28: e127–e129. 28. Loria R, Wedner H. Facial swelling secondary to inhaled bronchodilator abuse: catecholamine-induced sialadenosis. Ann Allergy 1989;62:289–293. 29. Seifert G. Tumour–like lesion of the salivary glands: the new WHO classification. Pathol Res Pract 1992;188:836–846. 30. Gao Y, Moonis G, Cunnane M, Eisenberg R. Lacrimal gland masses. AJR Am J Roentgenol 2013;201:W371–W381. 31. Jakobiec F, Yeo J, Trokel S, et al. Combined clinical and computed tomographic diagnosis of primary lacrimal fossa lesions. Am J Ophthalmol 1982;94:785–807. 32. Jakobiec F, Stagner A, Katowitz W, Eagle R Jr. A microanatomic abnormality of the lacrimal gland associated with Goldenhar syndrome. Surv Ophthalmol 2016;61:654–663. 33. Allen M, Wright P, Reid L. The human lacrimal gland. A histochemical and organ culture study of the secretory cells. Arch Ophthalmol 1972;88:493–497. 34. Paulsen F, Langer G, Hoffman W, Berry M. Human lacrimal gland mucins. Cell Tissue Res 2004;316:167–177. 35. Schäfer G, Hoffman W, Berry M, Paulsen F. Lacrimal glandassociated mucins. Age related production and their role in the pathophysiology of dry eye. Ophthalmologe 2005;102: 175–183. 282 36. Srinivasan D, Worgul B, Iwamoto T, Merriam G. The conjunctival epithelium II. Histochemical and ultrastructual studies on human and rat conjunctiva. Ophthalmic Res 1977;9: 65–79. 37. Bhattacharya D, Yu L, Wang M. Expression patterns of conjunctival mucin 5AC and aquaporin 5 in response to acute dry eye stress. PLoS One 2017;12:e0187188. 38. Roen JL, Stasior OG, Jakobiec FA. Aging changes in the human lacrimal gland: role of the ducts. CLAO J 1985;11: 237–242. 39. Schnoor M, Garcı́a Ponce A, Vadillo E, Pelayo R, Rossaint J, Zarbock A. Actin dynamics in the regulation of endothelial barrier functions and neutrophil recruitment during endotoxemia and sepsis. Cell Mol Life Sci 2017;74(11):1985–1997. 40. Jakobiec F, Perry H, Harrison W, Krebs W. Dacryoadenoma. A unique tumor of the conjunctival epithelium. Ophthalmology 1989;(96):1014–1020. 41. Bundele M, Weinreb I, Xu B, et al. Mucoacinar carcinoma: a rare intercalated duct/acinar variant of mucoepidermoid carcinoma: hybrid tumor or distinct entity? Lab Invest 2017; 97(S1):322A. abstract 1294. 42. Grossniklaus H, Abbuhl M, McLean I. Immunohistologic properties of benign and malgnant mixed tumors of the lacrimal gland. Am J Ophthalmol 1990;110:540–549. 43. Athwal H, Murphy G 3rd, Tibbs E, et al. Sox10 regulates plasticity of epithelial progenitors toward secretory units of exocrine glands. Stem Cell Rep 2019;12:366–380. 44. Brown F, Houston G, Lubow R, Sagan M. Adenomatoid hyperplasia of mucous salivary glands. Report of two cases. J Periodontol 1986;58:125–127. 45. Barrett A, Speight P. Adenomatoid hyperplasia of oral minor salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;79:482–487. 46. Bryant C, Manisali M, Barrett A. Adenomatoid hyperplasia of palatal minor salivary glands. J Larngol Otol 1996;110: 167–169. AMERICAN JOURNAL OF OPHTHALMOLOGY MAY 2020