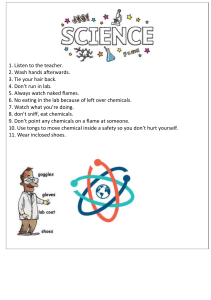
Steps for Doing Stoichiometry Example Problem 2H2O2 → 2H2O + O2 1. Balance the reaction. 2. Underline the two chemicals in the question that you will start and end the problem with. 3. Circle the same chemicals in the reaction. 4. Create a mol-to-mol ratio from the 2 chemicals that you just circled. 5. Start your conversion by writing out the given number in the question with units followed by a x ------------6. FIRST CONVERSION: Convert the starting number in the units of mols if needed. 7. SECOND CONVERSION: use the mol-to-mol ratio 8. THIRD CONVERSION: convert the answer to the units that the question asks for if needed. 9. Use your calculator to get an answer. How many grams of O2 can be formed by completely reacting 150.0g of H2O2? 2 𝑚𝑜𝑙𝑠 𝐻2𝑂 2 or 1 𝑚𝑜𝑙 𝑂2 1 𝑚𝑜𝑙 𝑂2 2 𝑚𝑜𝑙𝑠 𝐻2𝑂 2 150. 0 𝑔 𝐻2𝑂 × 2 1 𝑚𝑜𝑙 𝐻2𝑂 2 34 𝑔 𝐻2𝑂 2 × 1 𝑚𝑜𝑙 𝑂2 32 𝑔 𝑂 2 𝑚𝑜𝑙𝑠 𝐻2𝑂 2 × 1 𝑚𝑜𝑙 𝑂2 = 70.59 g O2 2 Mole Conversions