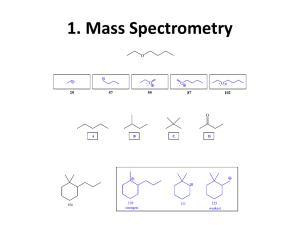
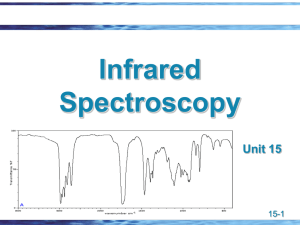
IR SPECTROSCOPY & ITS APPLICATIONS Amit Agnihotri Defence Reserach Laboratory Tezpur Spectroscopy is a technique used to determine the structure of a compound by the study of the interaction between matter and electromagnetic radiation Nondestructive (destroys little or no sample). EMR ANALYTE SPECTROPHOTOGRAPH 1.UV-Visible radiations--------excitation of electrons---------UV-visible spectrum 2.IR-radiations-----------------------vibration changes --------------------IR spectrum 3.Radio frequency---------------spin rotational changes-------------N.M.R spectrum THE ELECTROMAGNETIC SPECTRUM IR REGIONS Near infrared region RANGE 0.8-2.5 µ (12,500 - 4000 cm-1) Mid infrared region 2.5-15 µ (4000 - 667cm-1) Far infrared region 15-200 µ (667 - 50 cm-1) 3 TYPES OF SPECTROSCOPY UV- Vis spectroscopy uses electronic transitions to determine bonding patterns Infrared (IR) spectroscopy measures the bond vibration frequencies in a molecule and is used to determine the functional group. Nuclear magnetic resonance (NMR) spectroscopy analyzes the environment of the hydrogens in a compound. This gives useful clues as to the alkyl and other functional groups present. Mass spectrometry (MS) fragments the molecule and measures their mass. MS can give the molecular weight of the compound and functional groups PRINCIPLE OF IR SPECTROSCOPY Molecules are made up of atoms linked by bonds. The movement of atoms and the chemical bonds like spring and balls (vibration) This characteristic vibration are called Natural vibration. The energy of molecular vibration is quantized. When EMR (IR) is applied then it causes the vibration between the atoms of the molecules when, Applied infrared frequency = Natural frequency of vibration Then, Absorption of IR radiation takes place and a peak is observed. Different functional groups absorb characteristic frequencies of IR radiation. Hence gives the characteristic peak value. Therefore, IR spectrum of a chemical substance is a finger print of a molecule for its identification. Like a fingerprint no two unique molecular structures produce the same infrared spectrum. This makes infrared spectroscopy useful for several types of analysis. CRITERIA FOR A COMPOUND TO ABSORB IR RADIATION Change in dipole moment A molecule can only absorb IR radiation when its absorption cause a change in its electric dipole A polar bond is usually IR-active. A nonpolar bond in a symmetrical molecule will absorb weakly or not at all. MOLECULAR VIBRATIONS There are 2 types of vibrations: 1. Stretching vibrations 2. Bending vibrations 1. Stretching vibrations: Vibration or oscillation along the line of bond Change in bond length Occurs at higher frequency: 4000-1250 cm-1 2 types: a) Symmetrical stretching b) Asymmetrical stretching A) SYMMETRICAL STRETCHING: Both bonds increase or decrease in length simultaneously. H C H B) ASYMMETRICAL STRETCHING in this, one bond length is increased and other is decreased. H C H 2. BENDING VIBRATIONS Vibration or oscillation not along the line of bond • Also called as deformations • In this vibrations bond angle is altered • Occurs at low frequency : 1400-666 cm -1 • 2 types: a) In plane bending: E.g. scissoring, rocking b) Out plane bending: E.g. wagging, twisting • A) IN PLANE BENDING i. Scissoring: This is an in plane blending 2 atoms approach each other Bond angles are decrease H C H ii. Rocking: Movement of atoms take place in the same direction. H C H B) OUT PLANE BENDING i. Wagging: 2 atoms move to one side of the plane. They move up and down the plane. H C H ii. Twisting: One atom moves above the plane and another atom moves below the plane. H C H IR STRETCHING FREQUENCIES (ν) DEPEND ON? ν = frequency k = spring strength (bond stiffness) = reduced mass (~ mass of largest atom) Directly on the strength of the bonding between the two atoms (ν ~ k) Inversely on the reduced mass of the two atoms (v ~ 1/m) STRETCHING FREQUENCIES isolated C=C 1640-1680 cm-1 conjugated C=C 1620-1640 cm-1 aromatic C=C approx. 1600 cm-1 Conjugation lowers the frequency Frequency decreases with increasing atomic weight. Frequency increases with increasing bond energy. SUMMARY OF IR ABSORPTIONS 19 CLASSIFICATION OF IR BANDS Three types : strong (s), medium (m), or weak (w) Depending on their relative intensities in the IR spectrum. O—H AND N—H STRETCHING Both of these occur around 3300 cm-1, but they look different: Alcohol O—H is broad with rounded tip. Primary amine (RNH2) is broad with two sharp spikes Secondary amine (R2NH) is broad with one sharp spike. No signal for a tertiary amine (R3N) because there is no hydrogen. IR SPECTRUM OF ALCOHOLS IR spectrum of alcohols broad, intense O—H stretching absorption around 3300 cm-1. The broad shape is due to the hydrogen bonding interactions of alcohol molecules. IR SPECTRUM OF AMINES The IR spectrum of amines show a broad N—H stretching absorption centered around 3300 cm-1. IR SPECTRUM OF AMIDES strong absorption for the C═O at 1630–1660 cm-1. there will N—H absorptions at around 3300 cm-1. APPLICATIONS IDENTIFICATION OF SUBSTANCES To compare spectrums. No two samples will have identical IR spectrum. Criteria: Sample and reference must be tested in identical conditions, like physical state, temperature, solvent, etc. The “Fingerprint” Region (1200 to 600 cm-1) : Small differences in structure & constitution of molecule result in significant changes in the peaks in this region. Hence this region helps to identify an unknown compound. STUDYING PROGRESS OF REACTIONS Observing rate of disappearance of characteristic absorption band in reactants Rate of increasing absorption bands in products of a particular product. E.g. : O—H = 3600-3650 cm -1 , C=O = 1680-1760 cm-1 Measure the degree of polymerization in chemical compounds. DETERMINATION OF MOLECULAR STRUCTURE Used along with other spectroscopic techniques. Identification is done based on position of absorption bands in the spectrum.Eg.: C=O at 1717 cm-1. Absence of band of a particular group indicates absence of that group in the compd. DETECTION OF IMPURITIES Determined by comparing sample spectrum with the spectrum of pure reference compound. Eg.: ketone impurity in alcohols. Detection is favored when impurity possess a strong band in IR region where the main substance do not possess a band. Eg :Impurity in bees wax (with petroleum wax) MONITORING THE STRUCTURAL PLASTICITY OF PLANT CELL WALLS carbonyl band near 1700 cm−1indicates a high concentration in the older plant. higher protein content in the young plant is suggested by the negative amide I and amide II bands at 1650 and 1545 cm−1, respectively. there is a significant difference in the cellulosic nature of the two samples, with the older plant having a higher relative cellulose content PROTEIN QUANTITATION IR spectroscopy is one of the most well established techniques for the analysis of protein structure In protein amino acids are covalently linked via amide (peptide) bonds. it absorb in multiple regions of the mid-IR spectrum, By measuring amide bonds in protein chains, we can accurately quantifies an intrinsic component of every protein Amide A (about 3500 cm-1) is with more than 95% due to the N-H stretching vibration Amide I (between 1600 and 1700 cm-1) most intense absorption band in proteins. stretching vibrations of the C=O (70-85%) and C-N groups (10-20%) In order to determine protein and peptide concentration, the Spectrometer measures the intensity (peak height) of the Amide I band, NON-INVASIVE BLOOD GLUCOSE MONITORING Near Infrared spectroscopy is used across the ear lobe to measur e glucose Amount of near infrared light passing through the ear lobe depends on the amount of blood glucose in that region The ear lobe was chosen due to the absence of bone.tissues and also bec ause of its relatively small thickness OTHER APPLICATIONS 1. Determination of unknown contaminants in industry using FTIR. 2. Determination of cell walls of mutant & wild type plant varieties using FTIR. 3. Biomedical studies of human hair to identify disease states (recent approach). 4. Identify odor & taste components of food. 5. Determine atmospheric pollutants from atmosphere itself. 6. Examination of old paintings It is also used in forensic analysis in both criminal and civil cases, example in identifying polymer degradation, in determining the blood alcohol content etc. Chemical Analysis: Testing Pill Quality. According to "Medical News Today," scientists at the University of Maryland have been successful in using the method of near-infrared spectroscopy (NIR) to make a prediction regarding quick dissolution of pills inside the body. STRENGTHS AND LIMITATIONS IR alone cannot determine a structure. Some signals may be ambiguous. The functional group is usually indicated. The absence of a signal is definite proof that the functional group is absent. Correspondence with a known sample’s IR spectrum confirms the identity of the compound.