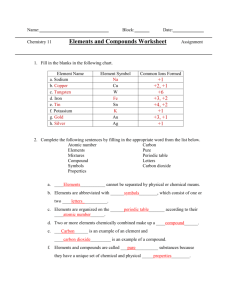
@ ! Binary Molecular Nomenclature 2. The first element keepsits name a. The first element gets a prefix if it has a subscript in the formula 3. The second element gets the -ide suffix (ending) a. The second element ALWAYSgets a prefix Compound Name Carbon dioxide N Compound Formula Carbon monoxide Diphosphorus pentoxide Dinitrogen monoxide Silicon dioxide Carbon tetrabromide | Sulfur dioxide Phosphorus pentabromide Y lodine trichloride Nitrogentriiodide Dinitrogentrioxide Compound Formula N20, SO3 NO NO> As205 PCI CCl, H20 SeFs à Compound Name 1- mono 2-di 3 — tri 4 — tetra 5 — penta 6 — hexa $ nonmetallic elements. Prefixes LEA 5 Rules for Binary Molecular Compounds 1. The naming system is for compounds composed of two Part 2A: Writing Binary Molecular Formulas Compound Name Carbon dioxide Compound Formula Carbon monoxide Diphosphorus pentoxide Dinitrogen monoxide Silicon dioxide Carbon tetrabromide Sulfur dioxide Phosphorus pentabromide lodine trichoride Nitrogen triiodide Dinitrogen trioxide Part 2B: Naming Binary Moiecular Compounds Compound Formula N20O4 SO; NO NO; As:0; PCI CCl, H20 Ser. Compound Name