Chemistry of Life: Atoms, Molecules, & Macromolecules
advertisement

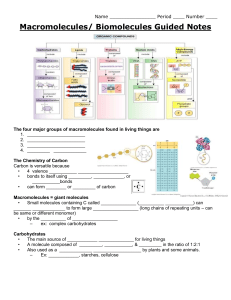
BIO.A.2.2 Describe and interpret relationships between structure and function at various levels of biochemical organization (i.e., atoms, molecules, and macromolecules.) BIO.A.2.1 Describe how the unique properties of water support life on Earth. BIO.A.2.3 Explain how enzymes regulate biochemical reactions within a cell. What are living creatures made of??? Living AND nonliving things are considered to be matter. Matter = Anything that has mass + takes up space The smallest unit of matter is an ATOM, therefore living things are made of atoms of different elements. ELEMENTS are made entirely of one type of atom! For example…the element calcium is made up of only calcium atoms! What elements are found in living things? 98% of living organisms is made of: Carbon (C) Hydrogen (H) Nitrogen (N) Oxygen (O) Phosphorus (P) Sulfur (S) How do these elements get inside living things? We eat, drink and breathe to take in more of these elements so they can be used for the following: to make more of us (cells) for growth for repair For example carbohydrates contain the carbon, hydrogen and oxygen needed by living things. How can nonliving ATOMS be used to make something that is living? Atoms of the elements C,H, N, O, P, S can be put together to make living things. The first step is to combine ATOMS to form MOLECULES! You must understand atomic structure before we can understand how molecules are assembled. Bonds There are different ways to bond atoms together, and each bond involves the interaction of electrons. Covalent bonds – atoms SHARE electrons Ionic bonds – atoms TRANSFER (gain or lose)electrons Examples: Covalent Bond Ionic Bond Molecules are formed by covalent bonds. What molecule is formed in the picture of the covalent bond above? Ions Ion: An atom that has gained or lost one or more electrons. Remember atoms are neutral overall; however if they lose or gain one or more electrons they become charged. Check out the examples below. Ions are important in living things. Cl 1- = chloride ion needed for brain function Na 1+ = sodium ion helps maintain electrical (nerve) impulses K 1+ = potassium ion helps maintain electrical (nerve) impulses Ca 2+ = calcium ion needed for muscle movement What is the difference between a calcium atom and a calcium ion? Sodium atom vs. sodium ion? Molecules MOLECULES are composed of two or more atoms held together by covalent bonds (bonds that involve the sharing of electrons). Can you name these common molecules found in living things? Biological Macromolecules Up to this point we have learned that atoms bonded together make molecules…So what do molecules bonded together form???? MACROMOLECULES Biological macromolecules are large molecules that are found in living things. They are necessary for living things to live and function. There are four main types of biological macromolecules, which include: 1.) Carbohydrates 2.) Proteins 3.) Lipids 4.) Nucleic Acids Building Macromolecules BIO.A.2.2.2 Describe how biological macromolecules form from monomers. How are biological macromolecules formed? Smaller molecules are bonded together to form bigger molecules in a process called SYNTHESIS! The smaller building block molecules are called monomers. The larger molecules that are formed are called polymers. Monomer Polymers Building Biological Macromolecules Carbohydrates = building blocks are simple sugars sugar – sugar – sugar – sugar – sugar – sugar Proteins = building blocks are amino acids amino amino amino amino amino amino acid – acid – acid – acid – acid – acid Nucleic acids = building blocks are nucleotides nucleotide – nucleotide – nucleotide – nucleotide Lipids = building blocks are fatty acids & glycerol fatty acid – fatty acid – fatty acid – fatty acid – fatty Dehydration Synthesis Vs. Hydrolysis Dehydration Synthesis Process that forms polymers Process used to make DNA, RNA, enzymes, antibodies, cellulose, hemoglobin, starch monomer + monomer polymer + H2O Ex.) building muscle, new cells, etc… Hydrolysis • Process that breaks down polymers • Polymer + H2O monomer + monomer • Ex.) digestion + Importance of Carbon BIO.A.2.2.1 Explain how carbon is uniquely suited to form biological macromolecules. The Importance of CARBON Carbon is an extremely important element because of its bonding capabilities. Carbon is able to form four bonds which makes it uniquely suited to form macromolecules. The macromolecules formed by carbon are usually: Large Complex (Rings & Chains) Diverse (Many functions) black atoms = carbon Let’s take a closer look….. Molecules w/ carbon = organic molecules Molecules without carbon = inorganic molecules Organic OR Inorganic: CO2 - Carbon dioxide NaCl - Table Salt O2 - Oxygen C6H12O6 - Glucose H2O - Water Are biological macromolecules (proteins, lipids, carbohydrates and nucleic acids) organic or inorganic? Biological Macromolecules BIO.A.2.2.3 Compare the structure and function of carbohydrates, lipids, proteins, and nucleic acids in organisms. Identifying Monomers by Structure What macromolecules will they form? Does it have a ring structure in its main chain? Carbohydrates (Polysaccharides) STRUCTURE Contain the elements: Carbon Hydrogen Oxygen Usually form a ring-like pattern. Carbs are made from monomers called simple sugars (monosaccharides). Examples include starch, cellulose, glycogen, polysaccharides (complex sugars) Found in foods such as breads, grains, vegetables, fruit and milk. FUNCTION • Provides energy for cell functions • Important component in plant cell structure & some animal cell structure STRUCTURE Contain the elements: Proteins Carbon Hydrogen Oxygen Nitrogen Sulfur (sometimes) Proteins are made up of monomers called amino acids. Amino acids have an amino group (-NH2) and a carboxyl group (-COOH)There are 20 different amino acids that are held together by special bonds called peptide bonds. Proteins form complex folded polypeptide chains. Examples include: Antibodies (part of the immune • • system) Hemoglobin (part of the blood) Enzymes (speed up chemical reactions like digestion) • • Found in foods such as meats, nuts, eggs and fish. • FUNCTION Help to build and repair cells. Help to speed up chemical reactions in living things. Help make antibodies (fight disease). Help transport molecules across plasma membranes. Found in muscles, hair, nails. Lipids Structure Function • Secondary source of energy for cells • Main component of plasma (cell) membranes • Help to insulate living things Contain the elements: Carbon Hydrogen Oxygen Usually form long straight chains or branching chains. Lipids are made from monomers of fatty acids and glycerol. Examples include: Oils Fats Cholesterol Triglycerides Phospholipids Waxes Structure Contain the elements: Carbon Hydrogen Oxygen Nitrogen Phosphorus Nucleic acids are made of monomers called nucleotides. Nucleotides contain a 5-carbon sugar, phosphate group (-PO4) and nitrogen base. There are 5 types of nucleotides: Adenine Guanine Cytosine Thymine Uracil Examples of nucleic acids include: DNA (deoxyribonucleic acid) RNA (ribonucleic acid) Nucleic Acids Function • DNA stores genetic information • RNA helps build proteins The Importance of Water BIO.A.2.1.1 Describe the unique properties of water and how these properties support life on Earth (e.g., high specific heat, adhesion, cohesion). Water AND Living Things Why is water important to living things? 1.) Water is necessary for aquatic ecosystems… Saltwater habitats Freshwater habitats 2.) Living things consist of approximately 70% water. Water helps to dissolve substances (vitamins, minerals, etc…) in living things. Water helps to transport substances (nutrients & waste) in living things. Water acts as a product or a reactant in many biochemical reactions such as photosynthesis, cellular respiration, dehydration synthesis and hydrolysis that occur in living things. Water helps maintain adequate temperature in living things. Enzymes rely on water in order to function properly in living things. Water Molecules Water is a polar molecule. Polar molecules have slightly charged regions. Water has a slightly negative oxygen region and two slightly positive hydrogen regions. Hydrogen Bonding Remember… a water molecule is held together by covalent bonds. Electrons are shared between the oxygen and hydrogen atoms of a water molecule. What holds one water molecule to another molecule??? The answer is…….HYDROGEN BONDS! Hydrogen bonds are bonds that form between a slightly positive hydrogen atom and another slightly negative atom. Where are the hydrogen bonds below? How many hydrogen bonds can be formed by one water molecule? Properties of Water Some properties of water are related to water’s ability to form hydrogen bonds. These properties of water include: High specific heat: resists changes in temperature Cohesion: attraction of water to other water molecules Adhesion: attraction of water molecules to different molecules Additional Properties of Water High Surface Tension: The molecules on the surface of a body of water are cohesive and form a surface “film.” High Heat of Vaporization: The amount of heat needed to turn a substance from a liquid state into its gaseous state. Maximum density at 4° C : Water is more dense as a liquid instead of a solid. (At what temp does water freeze? Does ice float or sink? Is ice more or less dense than liquid water?) Universal Solvent: Water is the most commonly used solvent (liquid used to dissolve other substances). Capillary Action: Cohesion and adhesion work together to draw a liquid up a narrow tube or opening. Identifying Properties of Water Identify which property/properties of water is displayed in each picture? Water = The Universal Solvent Solution: substance formed when one substance is dissolved in another substance. Solvent: substance that does the dissolving Solute: substance that gets dissolved solution Identify examples of solutes? Solvents? Solutions versus Suspensions Solutions (mix evenly throughout) Polar solvents dissolve polar solutes. Non-polar solvents dissolve non-polar solutes. Suspensions (do NOT mix evenly throughout) Polar solvents do NOT dissolve nonpolar solutes; therefore they separate. Non-polar solvents do NOT dissolve polar solutes; therefore they separate. pH of Solutions pH = measure of the concentration (amount) of hydrogen ions in a solution. Acid: releases a hydrogen ion when it dissolves in water. high H+ concentration pH less than 7 Base: releases hydroxide ions and removes hydrogen ions from a solution. low H+ concentration pH greater than 7 Why is pH important to living things? Most living things can only live in a narrow pH range. Enzymes cannot function properly when pH fluctuates too much. Most human cells range between 6.5 and 7.5. If pH fluctuates too drastically the chemical reactions within cells are altered. One way organisms control pH is through dissolved compounds called BUFFERS. Buffers are weak acids or weak bases that help neutralize strong acids and bases. Buffers help us maintain homeostasis by controlling pH. Review the handouts “Why is pH important to living things?” Enzymes BIO.A.2.3 Explain how enzymes regulate biochemical reactions within a cell. BIO. A.2.3.1 Describe the role of an enzyme as a catalyst in regulating a specific biochemical reaction. BIO.A.2.3.2 Explain how factors such as pH, temperature and concentration levels can affect enzyme function. Enzymes Enzymes are proteins that act as biological catalysts. (Catalysts are substances that speed up the reaction rate of chemical reactions.) Enzymes are usually named after the reaction they catalyze. They also typically end in –ase. For example: Lipase = enzyme which speeds the breakdown of lipids (fats) Protease = enzyme which speeds the breakdown of proteins DNA polymerase = enzyme which speeds the construction of the polymer DNA lipase protease Different structures = different functions Enzymes Enzymes are very specific, generally catalyzing only one chemical reaction. For example: The enzyme maltase speeds up the breakdown of maltose into separate glucose molecules. The enzyme maltase binds with the substrate (reactants = maltose) at a specific location called an active site in order to form glucose. Specific enzymes and substrates fit so perfectly together that they are referred to as a Lock and Key Model. Lock and Key Model Substrates bind to an enzyme at certain places called active sites. The enzyme brings substrates together and weakens their bonds. The catalyzed reaction forms a product that is released from the enzyme. Regulation of Enzyme Activity Enzymes play essential roles in controlling chemical pathways, making materials that cells need, releasing energy, and transferring information; therefore it is important to keep them functioning properly. A variety of factors affect the activity and ability of enzymes to function properly. These factors include: Temperature pH Concentration Enzymes function best in a narrow range of temperature, pH and concentration. If the temperature, pH or concentration fluctuates outside of this range the shape of the enzyme may change and the result is an enzyme incapable of performing its job. Remember…ENZYME FUNCTION DEPENDS ON ITS STRUCTURE!!!!