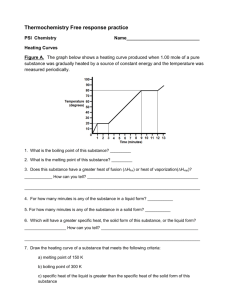
Thermochemistry Free response practice PSI Chemistry Name_____________________________ Heating Curves Figure A. The graph below shows a heating curve produced when 1.00 mole of a pure substance was gradually heated by a source of constant energy and the temperature was measured periodically. 1. What is the boiling point of this substance? _________ 2. What is the melting point of this substance? _________ 3. Does this substance have a greater heat of fusion (Hfus) or heat of vaporization(Hvap)? ____________ How can you tell? ________________________________________________ ____________________________________________________________________________ 4. For how many minutes is any of the substance in a liquid form? ___________ 5. For how many minutes is any of the substance in a solid form? ___________ 6. Which will have a greater specific heat, the solid form of this substance, or the liquid form? __________________ How can you tell? __________________________________________ ____________________________________________________________________________ 7. Draw the heating curve of a substance that meets the following criteria: a) melting point of 150 K b) boiling point of 300 K c) specific heat of the liquid is greater than the specific heat of the solid form of this substance The standard heats of formation of three hydrocarbons are given below. Substance Standard Heat of Formation, Hf o (kJ/mol) C2H2, acetylene +227 CH3OH, methanol -239 C2H5OH, ethanol -278 CO (g) -111 CO2(g) -394 H2O(l) -285 8) This is the balanced equation showing the complete combustion of acetylene: 2 C2H2 + 5 O2 (g) 4 CO2(g) + 2 H2O(l) a) Calculate the heat of reaction, H, in kJ. b) Calculate the heat of combustion,Hcomb, in kJ per mole acetylene (kJ/mol). c) Calculate the enthalpy change, H, when 1.00 g of acetylene C2H2, is completely burned. d) Is the enthalpy change in part (c) exothermic or endothermic? Justify your response. e) Suppose all of the heat you calculated (in part c) is applied to heat up a sample of water from 10oC to 95oC. What mass of water can be heated? Include units in your answer. The specific heat of water is = 4.18 J/g.oC. 9) Balance this equation showing the incomplete combustion of methanol: ___ CH3OH + ___ O2 (g) ___ CO(g) + ___ H2O(l) a) Calculate the heat of reaction, H, in kJ. b) Calculate the heat of combustion,Hcomb, in kJ per mole methanol (kJ/mol). c) Calculate the enthalpy change, H, when 4.5 g of methanol CH3OH, is completely burned. d) Is the enthalpy change in part (c) exothermic or endothermic? Justify your response. e) Suppose all of the heat you calculated (in part c) is applied to heat up a 1.0-kg (1000 g) sample of water. What will be the final temperature of the water, if it starts out at 22 oC ? The specific heat of water is = 4.18 J/g.oC. ANSWERS Figure A. The graph below shows a heating curve produced when 1.00 mole of a pure substance was gradually heated by a source of constant energy and the temperature was measured periodically. 1. What is the boiling point of this substance? 80o 2. What is the melting point of this substance? 20o 3. Does this substance have a greater heat of fusion (Hfus) or heat of vaporization(Hvap)? How can you tell? Hfus is greater because it takes 3 minutes to vaporize the sample whereas it takes only 2 minutes to melt the same amount of this substance. 4. For how many minutes is any of the substance in a liquid form? 11 minutes 5. For how many minutes is any of the substance in a solid form? 3 minutes 6. Which will have a greater specific heat, the solid form of this substance, or the liquid form? How can you tell? The liquid form of this substance has a greater specific heat. Since specific heat is proportional to the slope = 1/(mC), a steeper slope line segment has lower specific heat. The slope of the first segment is 20 whereas the slope of the segment as the liquid is heated has a slope of 10. 7. Draw the heating curve of a substance that meets the following criteria: a) melting point of 150 K b) boiling point of 300 K c) specific heat of the liquid is greater than the specific heat of the solid form of this substance Answers to Thermochemistry Free-Response Practice 8. a) -2600 kJ b) -1300 kJ/mol c) -50 kJ = 50,000 J d) exothermic, H <0 e) 141 g water 9. coefficients = 1, 1, 1, 2 a) -442 kJ b) same since coefficient is one, -442 kJ/mol c) -62 kJ = -62,000 J d) exothermic, H <0 e) T = 14.8 or 15 oC, so final temp is 36.8 or 37 oC