
MSE 280: Introduction to Engineering Materials Polymer Structures Reading: Callister Ch. 4 & 14.11 • • • • • • • • Classification. Monomers and chemical functional groups. Nomenclature. Polymerization methods. g & degree g of p polymerization. y Molecular weight Molecular structures. Copolymers. Crystallinity. 1 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Polymers -Very large molecules (macromolecules) that are composed of smaller repeating units (monomers). A A+A A-A A-A-A ….. - Examples: - Textile fibers: polyester, nylon… - IC packaging materials. - Resists for photolithography/microfabrication. - Plastic Pl ti b bottles ttl ((polyethylene l th l plastics). l ti ) - Adhesives and epoxy. - High-strength/light-weight fibers: polyamides, polyurethanes, Kevlar… - Biopolymers: DNA, proteins, cellulose… 2 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 1 Classification • Thermoplastics: polymers that flow more easily when squeezed, pushed, stretched, etc. by a load (usually at elevated l t d T) T). – Can be reheated to change shape. • Thermosets: polymers that flow and can be molded initially but their shape becomes set upon curing. – Reheating will result in irreversible change or decomposition. • Other ways to classify polymers. – B By chemical h i l ffunctionality ti lit ((e.g. polyacrylates, l l t polyamides, l id polyethers, polyeurethanes…). – Vinyl vs. non-vinyl polymers. – By polymerization methods (radical, anionic, cationic…). – Etc… 3 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Chemical functional groups H Ethylene (ethene) H C C H H H Propylene (propene) H = C C H C H H H 1-butene 2-butene trans Acetylene (ethyne) cis H C C H Saturated hydrocarbons Unsaturated hydrocarbons 4 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 2 Chemical functional groups 5 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Some common polymers teflon Polyacrylonitrile (PAN) H H C C H C N Note: many of these polymers have backbone. Vinyl polymers (one or more H’s of ethylene can be substituted) H H C C © 2007, 2008 Moonsub Shim, University of Illinois H X H H C C H X 6 nMSE280 3 Nomenclature Monomer-based naming: poly________ poly Monomer name goes here e.g. ethylene -> polyethylene if monomer name contains more than one word: poly( p y(_____ ____)) Monomer name in parentheses e.g. acrylic acid -> poly(acrylic acid) 7 © 2007, 2008 Moonsub Shim, University of Illinois polymethylene MSE280 Monomers to polymers polymerization ethylene polyethylene 8 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 4 Polymerization methods A. Free Radical Polymerization 1. Initiation H R H R C C Free radical initiator (unpaired electron) H H monomer H H C C H H Radical t transferred f d R R H H H C sp2 carbons C C H C H H H H σ bonds π bond © 2007, 2008 Moonsub Shim, University of Illinois sp3 carbon 9 MSE280 Polymerization methods A. Free Radical Polymerization H 2. Propagation H R H C C H H H H R C C H H H C C H H H H H C C C C H H H H H R H H H H H H C C C C C C H H H H H H H H C H R H H C C C H H H © 2007, 2008 Moonsub Shim, University of Illinois Both carbon atoms will change from sp2 to sp3. 10 MSE280 5 Polymerization methods A. Free Radical Polymerization 3. Termination R R H H C C H H H H C C H H + R + R R H H C C H H H H C C R H H R H H H H C C C C H H H H R Intentional or unintentional molecules/impurities can also terminate. 11 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Polymerization methods Lose water (condensation) B. Stepwise polymerization O O H2N R C + OH H2N R C O OH H2N R C N H R C OH + H H N R Various R groups… n H O Proteins (polypeptides have similar composition) O H N H C C O O R + (n-1) C H O H n C. Other methods Anionic polymerization, cationic polymerization, coordination polymerization… © 2007, 2008 Moonsub Shim, University of Illinois 12 MSE280 6 Molecular Weight Not only are there different structures (molecular arrangements) but there can also be a distribution of molecular weights (i.e. number of monomers p per p polymer y molecule). ) 16 mers 20 mers 10 mers Average molecular weight = 20 + 16 + 10 M monomer = 15.3M monomer 3 This is what is called number average molecular weight. © 2007, 2008 Moonsub Shim, University of Illinois 13 MSE280 Molecular Weight Number average molecular weight: ∑N M = ∑N j Mn j j j j = mo ∑ N j j j ∑N j j Nj = number of polymer chains with length j Mj = jmo = mass of polymer chain with length j mo = monomer molecular weight g Note: ∑N M j j j = Total weight ∑N j = Total # of polymer chains j 14 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 7 Molecular Weight Weight average molecular weight: ∑W M = ∑W j Mw ∑N M = ∑N M j j 2 j j j Wj = N jM j j j j In general: j j ∑N Mα M= ∑N Mα +1 j j j j j j If α = 0 then M n If α = 1 then M w 15 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Molecular Weight: Different Notations In Callister Textbook In Lecture Notes ∑N M = ∑N j Mn M n = ∑ xi M i j i j xi = j j Mw = ∑ N j M 2j j ∑N j M Ni ∑N j j M w = ∑ wi M i i j j Nj = number of polymer chains with length j Mj = jmo = mass of polymer chain with length j © 2007, 2008 Moonsub Shim, University of Illinois wi = Ni M i ∑ N jM j j 16 MSE280 8 Molecular Weight Weight average counts the heavier chains more – certain properties can be dependent on molecular weight (i.e. larger MW polymer chains can contribute to the overall property differently than smaller ones). NOTE: Mw is always larger than Mn unless polydispersity = 1 17 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Polydispersity & Degree of polymerization Polydispersity = Mw ≥1 Mn When polydispersity = 1, monodisperse. Degree of polymerization: Number avg degree of polymerization Weight avg degree of polymerization © 2007, 2008 Moonsub Shim, University of Illinois nn = nw = Mn mo Mw mo Monomer molecular weight 18 MSE280 9 Example 1 A. Calculate number and weight avg degrees of polymerization and polydispersity for a polymer sample with the following distribution. Avg # of monomers/chain 10 100 500 1000 5000 50,000 Relative abundance 5 25 50 30 10 5 B. If the polymer is PMMA, calculate number and weight average molecular weights. C. If we add polymer chains with avg # of monomers = 10 such that their relative abundance changes from 5 to 10, what are the new number and weight average degrees of polymerization and polydispersity? 19 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Isomerism in polymers So far, we’ve considered polymers as if they were beads on a string, there is more structural complexity. Isomers: molecules with same empirical formula but different structure. geometrical isomers: different sequence of bonds. H Cl H C C Cl H H Cl H Cl C C H H H stereoisomers: same bond sequence but different arrangementt off atoms t in i space. Cl H Cl H Cl C C C C Cl H H Similar to molecules polymers also have isomers… sequence, stereo and structural isomers © 2007, 2008 Moonsub Shim, University of Illinois 20 MSE280 10 Sequence isomerism For an asymmetric monomer T + H T H F H H F H H H C C C C C C C C H H H F H H H F H to T H T H T H H T H T T H e.g. PMMA e.g. poly(vinyl fluoride): H T H H3C H3C H3C O O O O O O O C H C H C H C C C C C C C H CH3 H CH3H CH3 H H3C T to T O C C CH3 H to H H to T Random arrangement H to T H to T Exclusive H to T arrangement (Why?) 21 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Stereoisomerism (tacticity) Even with exclusive head-to-tail arrangement, there can still be variations R H C H H C C or H X H H H HH X H X H meso-diad © 2007, 2008 Moonsub Shim, University of Illinois C H X H H HH X H H X racemic-diad 22 MSE280 11 Stereoisomerism (tacticity) Isotactic: only meso-diads X X X X X X X Syndiotactic: only racemic-diads X X X Atactic: random mixture X X X X X 23 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Structural Isomers Some polymers contain monomers with more than 1 reactive site e.g. isoprene 2 1 H2C 4 CH3 C C H CH2 3 trans-isoprene trans-1,4-polyisoprene CH3 C H2 C C H trans-1,2-polyisoprene H2 C H2 C C n H3C n 3,4-polyisoprene H2 C H C CH H2C C H2C Note: there are also cis-1,4- and cis-1,2-polyisoprene © 2007, 2008 Moonsub Shim, University of Illinois n CH3 24 MSE280 12 Molecular structure So far, we’ve considered polymers as simple linear chain of one type of monomer… Linear structures a) Linear homopolymer: …--A—A—A—A—A—A—A—A—A—A—A—A—A—A—A--… b) Linear copolymer: …--A—B—A—B—A—B—A—B—A—B--… A B A B A B A B A B alternating copolymer …--A—A—A—A—A—B—B—B—B—B--… block copolymer …--A—B—B—A—B—A—A—A—B—B--… random copolymer 25 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Molecular structure Star branching © 2007, 2008 Moonsub Shim, University of Illinois -B-B-B B-B-B-B-… Long branching -B-B-B B-B-B-B-… Short branching …A-A-A-A-A-A-A-A-A-A-A-… -B-B-B B-B-B-B-… Branched structures Graft copolymer Dendrimers 26 MSE280 13 Molecular structure Secondary bonding between chains secondary Covalently linked chains bonding Linear Branched Cross-Linked Network Direction of increasing strength From Callister resource CD Not always in this order (e.g. high-density vs. low-density polyethylene). Why? 27 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Molecular structure How do crosslinking and branching occur in polymerization? 1. Start with or add in monomers that have more than 2 sites that bond with other monomers. e g crosslinking polystyrene with divinyl benzene e.g. … … … stryene Control degree of crosslinking by styrenedivinyl benzene ratio … polystyrene … … + styrene divinyl benzene crosslinked polystyrene Monomers with three or more polymerizable groups lead to network polymers. 28 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 14 Molecular structure Branching in polyethylene (back-biting) H2C CH2 R H2 C C H2 H2 C C H2 Same as H H C H CH2 C CH2 R H2 C C H H H Radical moves to a different carbon (H transfer) C H H2 H C H CH2 C R CH2 C H H2 Polymerization continues from this carbon This process is usually difficult to avoid and leads to low-density polyethylene (highly branched). When there is small degree of branching: high-density polyethylene. 29 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Molecular structure copolymers Block Alternating Random Graft 30 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 15 Example 2 A nitrile rubber copolymer, co-poly(acrylonitrilebutadiene) is found to have butadiene), M n = 106,740 g / mol and nn = 2000 Calculate the ratio of number of acrylonitrile y to number of butadiene. 31 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Crystalline or Amorphous We’ve seen crystalline and amorphous materials in metals and ceramics. What about polymers? © 2007, 2008 Moonsub Shim, University of Illinois 32 MSE280 16 Polymer crystallinity Some are amorphous, some are partially crystalline (semi-crystalline). -Why is it difficult to have a 100% crystalline polymer? %crystallin t lli ity it = ρc ( ρ s − ρ a ) ×100% ρ s (ρc − ρa ) ρs = density of specimen in question ρa = density of totally amorphous polymer ρc = density of totally crystalline polymer 33 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Polymer crystallinity %crystallinity = M crystalline M total ×100% = ρ cVc ρ ×100% = c f c × 100% ρ sVs ρs What is fc? Volume fraction of crystalline component. To get fc : M total= M crystalline + M amorphous Ms = Mc + Ma ρ sVs = ρ cVc + ρ aVa ρ s = ρc Vc V + ρa a Vs Vs 34 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 17 Polymer crystallinity Using definition of volume fractions: fc = Vc Vs and fa = ρ s = ρ c f c + ρ a f a = ρ c f c + ρ a (1 − f c ) ρ s = fc (ρc − ρa ) + ρa ρ − ρa fc = s ρc − ρ a Va Vs Substituting g in fc into the original g definition,, we get: g %crystallinity = ρc ⎛ ρ s − ρa ⎞ ⎜ ⎟ ×100% ρ s ⎜⎝ ρ c − ρ a ⎟⎠ 35 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Polymer crystallinity Degree of crystallinity depends on processing conditions (e.g. cooling rate) and chain configuration. Cooling rate: during crystallization upon cooling through MP, polymers become highly viscous. Requires sufficient time for random & entangled chains to become ordered in viscous liquid. Chemical groups and chain configuration: More Crystalline Less Crystalline Smaller/simper S ll / i side id groups L Larger/complex / l side id groups Linear Highly branched Crosslinked, network Isotactic or syndiotactic Random 36 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 18 Semicrystalline polymers Fringed micelle model: crystalline region embedded in amorphous region. A single chain of polymer may pass through several crystalline regions as well as intervening amorphous regions. 37 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 Semicrystalline polymers Chain-folded model: regularly shaped platelets (~10 – 20 nm thick) sometimes forming multilayers. Average chain length >> than platelet thickness. 38 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 19 Semicrystalline polymers Spherulites: Spherical shape composed of aggregates of chainfolded cystallites. Natural rubber Cross-polarized light through spherulite structure of polyethylene. © 2007, 2008 Moonsub Shim, University of Illinois 39 MSE280 Diblock copolymers F. Bates, Science 1991. 40 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 20 Concepts to remember… • • • • • • • • • Classification: thermosets and thermoplastics. Monomers and chemical functional groups. Nomenclature. Polymerization methods: free radical, addition, and other polymerization. Number & weight average molecular weights, polydispersity & degree of polymerization. Sequence isomerism, tacticity, structural isomerism Molecular structures: linear, branched, crosslinked, network… Copolymers: block, alternating, random and graft. Crystallinity: fringed micelles & chain-folded models and spherulites. 41 © 2007, 2008 Moonsub Shim, University of Illinois MSE280 21

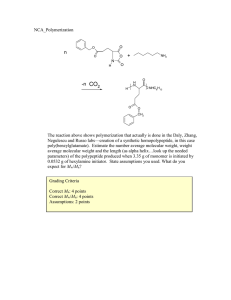


![\t<L Your Name: _[printed]](http://s2.studylib.net/store/data/013223479_1-5f2dc062f9b1decaffac7397375b3984-300x300.png)