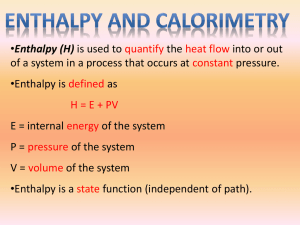
Enthalpy of Chemical Reactions Out main interest here is to apply the first law of thermodynamics to chemical reactions carried out under certain conditions. These conditions are constant volume and constant pressure. This is because when we carry out the experiment in the laboratory, we usually work with definite volume (constant volume) of reaction. In addition, the pressure inside the laboratory is pretty much the same as the atmospheric pressure, which is constant (constant pressure). Thus we have two different kinds of quantities, ∆E and ∆H, to measure the energy changes one at constant volume (∆E) and another at constant pressure (∆H). Both are thermodynamic state functions; ∆E is known as the change in internal energy and ∆H is labeled as the change in enthalpy (from Greek, enthalpein, to warm). Remember, the word enthalpy is simply a fancy word for heat at constant pressure. Enthalpy Change in Reactions Since most of the chemical reactions in laboratory are nothing but the constant-pressure processes, we can write the change in enthalpy (also known as enthalpy of reaction) for a reaction. Consider the following general type of reaction reactant Æ products The ∆H is defined as the difference between the enthalpies of products and the reactants. Thus ∆H = Hproducts - Hreactants The enthalpy of reaction can be positive or negative or zero depending upon whether the heat is gained or lost or no heat is lost or gained: ∆H > 0, if Hproducts > Hreactants endothermic reaction (∆H is positive (+)) ∆H < 0, if Hproducts < Hreactants, exothermic reaction ∆H = 0, if Hproducts = Hreactants, no heat is lost or gained (∆H is zero) (∆H is negative (-)) Thermochemical Equations When we write chemical equations to represent chemical reactions, we simply write the balanced chemical equations. However, within the realm of the thermodynamics, we must write the chemical equations with change in heat (enthalpy change). Such equations are known as thermochemical equations. Consider the process of melting the ice, for which we write the following chemical equation: H2O (s) Æ H2O (l) 1 This equation simply says that one mole of ice melts and one mole of water forms. But in reality, the melting process involves absorption of certain amount of heat by the ice. Now we have to incorporate that heat as a part of the chemical reaction that is indicated by the enthalpy change. When you do that it becomes a thermochemical equation. H2O (s) Æ H2O (l) ∆H = 6.01 kJ/mol First of all, the ∆H is positive (∆H >0) because it is an endothermic reaction (heat is absorbed by the ice from the surrounding), and secondly, ∆H is written per mole of reaction. In this example, 1 mole of ice is converted to 1mole of water. As another example, consider the oxidation of graphite to produce carbon dioxide gas: C (graphite) + O2 (g) Æ CO2 (g) ∆H = - 393.5 kJ/mol Here the ∆H is negative (∆H <0) because it is an exothermic reaction. It means that when 1 mole of graphite is burned, 393.5 kJ heat is liberated to the surrounding. The above written equations for the melting of ice and oxidation of graphite are examples of thermochemical equations that show mass relationship as well as enthalpy change. There are certain properties of thermochemical equations that you should know: 1. You must always specify the physical state of all reactants and products because it helps to determine the actual enthalpy change. As you know the chemical formula for water is H2O whether it is in solid state (ice) or liquid state (water) or gaseous state (vapor). However, the amount of energy involved going from one state to another state is not the same. The above mentioned 6.01 kJ refers to the enthalpy change going from the solid state to the liquid sate only. 2. If we multiply both sides of the thermochemical equation by a certain number, then the ∆H must also be multiplied by the same number. Thus if we multiply the melting of ice equation by 2, then we also have to multiply the ∆H by 2. 2H2O (s) Æ 2H2O (l) ∆H = 2 x 6.01 kJ/mol = 12.02 kJ/mol 3. When we reverse the thermochemical equation, we make the reactants to become products and products to become reactants. In that case, the sign on ∆H must be reversed without changing the magnitude of ∆H. For examples, freezing of water to ice is represented by H2O (l) Æ H2O (s) ∆H = - 6.01 kJ/mol This now becomes an exothermic reaction. This makes sense because when the ice is formed the heat is released (lost) to the surrounding by the water. If the water does not lose the heat, it will not become an ice. 2 Example-1 Consider the reaction H2 (g) + Cl2 (g) Æ 2HCl (g) ∆H = -184.6 kJ/mol What is the value of ∆H if (a) the equation is multiplied throughout by 3, and (b) the direction of the reaction is reversed so that the products become the reactants and vice versa. Answer-1 (a) When you multiply the equation by 3, the ∆H must also be multiplied by 3. Thus 3H2 (g) + 3Cl2 (g) Æ 6HCl (g) ∆H = 3 x -184.6 kJ/mol = - 553.8 kJ (b) If the direction of reaction is reversed, the sign on ∆H is reversed without changing the magnitude. Thus 2HCl (g) Æ H2 (g) + Cl2 (g) ∆H = 184.6 kJ/mol Example-2 Given the following thermochemical equation CH4 (g) + 2O2 (g) Æ CO2 (g) + 2H2O (l) ∆H = -890.4 kJ/mol calculate the heat evolved when 20 g of CH4 is converted to CO2 (g) and H2O(l). Answer-2 The chemical equations are always written in terms of moles, but problem is stated in terms of mass. You need to relate mole to mass through molar mass (mole = mass / molar mass). There are two ways of doing this problem; both should yield the same result. 1. moles of CH4 Æ grams of CH4 Æ kilojoules of heat 2. grams of CH4 Æ moles of CH4 Æ kilojoules of heat First Method: First calculate the mass of one mole of CH4, which is 16 g/mol. Then set up the ratio to calculate the heat produced by 20 g of CH4. 20 g CH 4 x 1mol CH 4 −890.4kJ x = −1113.0kJ 16 g CH 4 1mole CH 4 3 Second Method: First convert 20 g of CH4 to moles of CH4, that is mol CH 4 = 20 g CH 4 = 1.35 mol CH 4 16 g CH 4 Then, set up the ratio to calculate the heat produced by 1.25 mol of CH4. 1.25 mol CH 4 x −890.4kJ = −1113.0kJ 1mol CH 4 Notice the similarity between both setups. The factor, 20 g CH 4 x method is nothing but 1.25 mol CH4. 4 1mol CH 4 , in the first 16 g CH 4