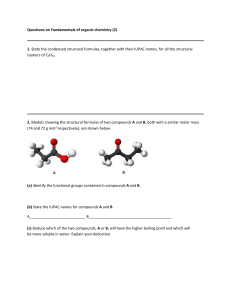
Topic 4 Review Questions [25 marks] 1. Which compound has the shortest C to O bond? [1 mark] A. CH3CHO B. CO C. CO2 D. C2H5OC2H5 2. Which describes a resonance structure? [1 mark] A. Double bond can be drawn in alternative positions. B. Bonds vibrate by absorbing IR radiation. C. A double and a single bond in the molecule D. A Lewis structure 3. What is the structure and bonding in SiO2 (s)? [1 mark] 4. Which is correct for all solid ionic compounds? [1 mark] A. High volatility B. Poor electrical conductivity C. Low melting point D. Good solubility in water 5. Which combination corresponds to a strong metallic bond? [1 mark] 6. What is the order of increasing boiling point? [1 mark] A. CH3CH2CH2CH3 < CH3CH(OH)CH3 < CH3COCH3 < CH3CO2H B. CH3CH2CH2CH3 < CH3COCH3 < CH3CH(OH)CH3 < CH3CO2H C. CH3CO2H < CH3COCH3 < CH3CH(OH)CH3 < CH3CH2CH2CH3 D. CH3CH2CH2CH3 < CH3COCH3 < CH3CO2H < CH3CH(OH)CH3 7. Which combination causes the strength of metallic bonding to increase? [1 mark] 8. Which molecule contains an incomplete octet of electrons? [1 mark] A. NF3 B. BF3 C. BrF D. SF2 9. Which compound has hydrogen bonds between its molecules? A. CH4 B. CH4O C. CH3Cl D. CH2O [1 mark] 10. How many lone pairs and bonding pairs of electrons surround the central [1 mark] chlorine atom in ClF2+? 11. Which molecule is polar? [1 mark] A. BeCl2 B. BCl3 C. NCl3 D. CCl4 12. Which species has the same molecular geometry as SO32−? [1 mark] A. BF3 B. SO3 C. PF3 D. CO32− 13. Which form of carbon is the poorest electrical conductor? A. Graphite B. Graphene C. Diamond D. Carbon nanotube [1 mark] 14. What is the molecular geometry and bond angle in the molecular ion NO 3−? [1 mark] 15. What is the formula of ammonium phosphate? [1 mark] A. (NH3)3PO4 B. (NH4)3PO4 C. (NH4)2PO4 D. (NH3)2PO3 16. What are the predicted electron domain geometries around the carbon and both nitrogen atoms in urea, (NH2)2CO, applying VSEPR theory? [1 mark] 17. The compounds shown below have similar relative molecular masses. What is the correct order of increasing boiling point? [1 mark] A. CH3COOH < (CH3)2CO < (CH3)2CHOH B. CH3COOH < (CH3)2CHOH < (CH3)2CO C. (CH3)2CO < CH3COOH < (CH3)2CHOH D. (CH3)2CO < (CH3)2CHOH < CH3COOH 18. What is the formula of magnesium nitride? A. MgN B. Mg2N3 C. Mg3N D. Mg3N2 [1 mark] 19. Which of the following series shows increasing hydrogen bonding with water? [1 mark] A. Propane < propanal < propanol < propanoic acid B. Propane < propanol < propanal < propanoic acid C. Propanal < propane < propanoic acid < propanol D. Propanoic acid < propanol < propanal < propane 20. The electronegativity values of four elements are given. [1 mark] What is the order of increasing polarity of the bonds in the following compounds? A. CO < OF2 < NO < CF4 B. CF4 < CO < OF2 < NO C. NO < OF2 < CO < CF4 D. CF4 < NO < OF2 < CO 21. Which two atoms form the most polar bond? A. C and F B. C and Cl C. Si and F D. Si and Cl 22. Which correctly states the strongest intermolecular forces in the compounds below? [1 mark] [1 mark] 23. How many bonding electrons are there in the urea molecule? A. 8 B. 16 C. 20 D. 24 24. Which compound contains both ionic and covalent bonds? [1 mark] [1 mark] A. SIH4 B. NaNO3 C. H2CO D. Na2S 25. Which substance has a giant covalent structure? [1 mark] © International Baccalaureate Organization 2020 International Baccalaureate® - Baccalauréat International® - Bachillerato Internacional® Printed for United Nations International school