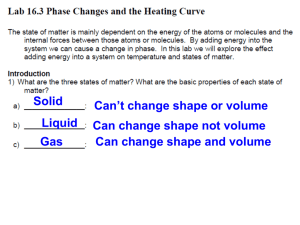
8 Science Changing States of Water Aim To investigate the effect of heat on the three states of water. Materials Time (min) 0 1 2 3 4 5 6 7 8 Beaker Ice Temp oC Time (min) 9 10 11 12 13 14 15 16 17 Retort stand/boss head/clamp Thermometer Stop watch Temp oC Time (min) 18 19 20 21 22 23 24 25 26 Temp oC Time (min) 27 28 29 30 31 32 33 34 35 Temp oC Method 1. Set up your equipment as demonstrated by your teacher. You can share a hotplate with another group. 2. Add ice to the beaker with some water. 3. Place the thermometer in the ice (not touching the bottom) and wait until the alcohol stops moving (hopefully at about 0 oC). 4. Turn on the hot plate and take a reading every minute. In your books Record your observations of the ice water as it is heated. Does the temperature increase much whilst the ice is melting? What happens to the rate of temperature increase after the ice has melted? At what temperature did the water start boiling? What happened to the rate of temperature increase as the water boiled? Explain why the temperature stopped increasing after the water was boiling vigorously. Draw, on graph paper a set of results. Describe what happens to water as it is heated, using your graph. Explain what is happening to water at key points on your graph.