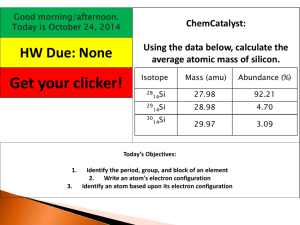
Name ______________________________ Period _____ Date _______________ CHEMISTRY I ATOMIC STRUCTURE REVIEW PART I: Scientists Match the following scientist to the correct contribution. A. Dalton B. Thomson C. Bohr D. Rutherford E. Aristotle F. Democritus _____1. used gold foil experiment to determine that most of the atom is empty space and that there was a small, dense core inside the atom _____2. developed the atomic theory: _____3. experimented with cathode ray tube and discovered the electron and _____4. proposed a general theory that the world was made of empty space and small, indivisible particles called atomos; not based on experimentation _____5. originated the idea that electrons travel in definite energy levels around the nucleus _____6. matter was made of hyle, not atoms. Atomic Theory (Postulates) 1. All elements are composed of _____________________ particles called ___________________________. 2. Atoms of the same element are _________________________. The atoms of any one element are _________________________ from those of another. 3. Atoms of different elements mix or combine in whole number _____________________________. 4. Chemical reactions occur when atoms ___________________, join, or _______________________________. In a chemical reaction, atoms of one element NEVER ___________________ into another. PART II: Subatomic Particles Complete the following chart. Particle Symbol Charge Relative Mass Location Proton Neutron Electron PART III: Atomic Structure Fill in the blanks. 1. ATOMIC NUMBER = __________________ = ___________________ 2. MASS NUMBER = _____________________ + ____________________ 3. NUMBER OF NEUTRONS = ___________________ - __________________ 4. Complete the following chart. Element Protons ** Boron Neutrons 6 70 ** Carbon ** Electrons 5 50 Mass Number 12 20 19 12 21 39 14 5. Write nuclear symbols for all of the elements in the chart above. 6. Define an isotope. 7. How are carbon-12 and carbon-14 similar? How are they different? 7. Draw Bohr models of those marked **. 8. Complete the following table. Element Symbol At. number Protons Neutrons oxygen-16 8 oxygen-17 8 9 oxygen-18 8 nitrogen-15 8 Ne beryllium-9 4 Average Atomic Mass Electrons Mass number 16 18 20 1. What is the atomic mass of silicon if 92.21% of its atoms have a mass of 27.977 amu, 4.70% have a mass of 28.976 amu, and 3.09% have a mass of 29.974 amu? (28.09 amu) 2. Calculate the average atomic mass of chromium, given the percent abundance and mass of each isotope: 4.350 % at 49.946 amu; 83.790% at 51.941 amu; 9.500% at 52.941 amu; and 2.360% at 53.939 amu. (52.00 amu) 3. Precise atomic masses of each isotope of magnesium are given below, along with the fractional abundance of each isotope. Calculate the average atomic mass. (24.31 amu) isotope Mg-24 Mg-25 Mg-26 mass (amu) 23.98504 24.98584 25.98259 Percent abundance 78.70 % 10.13 % 11.17 %