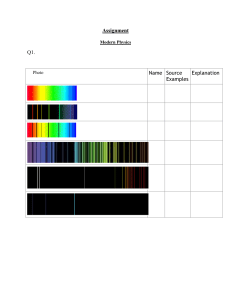
School of Physics & Astronomy PHS1022 Quantum Physics - Sample Problem Set 1 QUESTION 1 (a) Monochromatic light of wavelength 300 nm is incident normally on a sodium (Na) surface of area 4 cm2. If the intensity of the light is 0.15 Wm-2, determine the rate at which photons strike the Na surface. Solution The energy per photon is: The power (or energy flux) is given by: . Hence, the rate at which photons strike the Na surface is: (b) What is the range of kinetic energies of photoelectrons emitted from the Na surface when illuminated by light of wavelength 300 nm? Hint: The work function of Na is 2.28 eV. Solution The maximum kinetic energy is determined from Einstein’s photoelectric equation: In the photoelectric effect an electron with the highest amount of energy in the metal absorbs a photon of energy hf. The kinetic energy of these “surface” electrons is given by the Einstein equation, where the work function, W, represents the minimum amount of energy necessary to remove an electron from the surface of the metal. However, it is possible for electrons to be emitted with kinetic energies less than Kmax. These electrons come from lower energy electron states in the metal, where they are bound more tightly. Therefore photoelectrons can emerge from the surface with 0 eV this corresponds to the minimum kinetic energy. QUESTION 2 Consider an antihydrogen atom, which consists of an antielectron (i.e., a positron, ) orbiting an antiproton The masses of the positron and the antiproton are identical to that of an electron and proton, respectively. However, the positron has a charge +e and the antiproton has a charge -e. (a) Calculate the radius of the first Bohr orbit in the antihydrogen atom. Solution Note that since we have simply interchanged a positron for an electron and an antiproton for a proton, the quantum mechanics of an antihydrogen atom is expected to be identical to that of a hydrogen atom. We can therefore utilise the results of the Bohr model of a H-atom directly. The radius of the first Bohr orbit (n = 1) is given by: where Z = 1 (the “nucleus” consists of a single negative charge), and the reduced mass for antihydrogen is: (b) Calculate the ionization energy in eV for an antihydrogen atom in the ground state. Solution Using the reduced mass M and Z = 1, we have: The ionization energy is then given by: (c) Calculate the wavelength of the photon emitted when an antihydrogen atom makes a transition from the n = 2 state to the n = 1 state. To what part of the electromagnetic spectrum does this radiation correspond? Solution Using the expression for the energy obtained in part (b) we write: The difference in energy between the two states is: The wavelength is therefore given by: This lies within the ultraviolet (UV) part (< 400 nm) of the electromagnetic spectrum. QUESTION 3 Figure 1 shows a schematic representation of a Young’s double slit experiment that uses electrons. The source produces monoenergetic electrons with a fixed energy. A moveable detector D is used to record the number of electrons arriving at a point x on a distant screen. Screen Double Slit Path 2 D Slit 2 x Electron Source Path 1 d 0 Slit 1 L Figure 1: Young’s double slit experiment with electrons. (a) Discuss briefly the experimental phenomenon of electron diffraction and its interpretation in terms of quantum physics. Solution A beam of collimated electrons is sent through a double slit (see Figure 1). An interference pattern consisting of maxima and minima is observed when a large number of electrons are recorded (e.g., 70,000 electrons were recorded in the Tonomura experiment, see e.g., http://www.hqrd.hitachi.co.jp/global/doubleslit.cfm). The fringes exhibit a interference pattern that is characteristic of the Young’s two slit experiment with light. Here , where is the de Broglie wavelength of the monoenergetic electrons. The arrival of the electrons at the detector (screen) is random (non-deterministic), and the electrons are localised on detection as one would expect from a particle; however, the fringe pattern is only determined correctly if one attributes a de Broglie wavelength to the electrons, in which case constructive or destructive interference between the electron wavefunctions occurs depending on their phase (path) difference. The probability of detection is determined by superposing the wavefunctions as indicated in part (b) below. A full discussion of electron diffraction can be found in the PHS1022 Quantum Physics Workshops that can be accessed via Moodle. (b) Write an expression for the probability of detecting an electron at position x, between x and x+ dx, on the distant screen. Take care to define all symbols. Solution According to quantum physics the probability of detecting an electron at a point x between x and x + dx, is given by: Here denotes the wavefunction that describes an electron passing through slit 1, travelling over path 1 and being detected at D. Similarly, is the wavefunction describing an electron passing through slit 2, travelling over path 2 and being detected at D. Note that if the electron wavefunctions are in phase (i.e., the probability of detection is a maximum. However, if the wavefunctions are out of phase (i.e., the probability of detection is a minimum. The quantum expression for the probability distribution exhibits interference (maxima and minima) in the distribution of electrons. The interference terms become apparent if we expand the expression for the probability, i.e., Here φ denotes the phase difference between the electron wavefunctions (c) and In what way does the probability obtained in (b) differ from the predictions of classical physics? Solution According to classical physics there is no interference between the electrons. The probability of detecting an electron at the distant screen is given classically by: where the wavefunctions are defined in (b). The classical expression represents the sum of the probabilities, without the interference terms. Hence in classical physics there is no possibility of obtaining a Young’s interference pattern with a double slit! _____________________ School of Physics & Astronomy PHS1022 Quantum Physics - Sample Problem Set 2 QUESTION 1 A beam of X-rays from a synchrotron is incident on a vessel containing gaseous krypton (Kr). Figure 1 shows the X-ray absorption spectrum near the K-edge of Kr. Figure 1: X-ray absorption spectrum for gaseous Kr. (a) Calculate the wavelength (in nm) of X-rays at the absorption K-edge of Kr. Solution From Figure 1 the absorption K-edge of krypton occurs at approximately Equating the K-edge energy with the photon energy we have: Thus the wavelength of X-rays corresponding to the K-edge in krypton is 0.113 nm. (b) The power in the synchrotron beam at the energy corresponding to the K-edge is 7.3W. Calculate the number of X-ray photons per second incident on the vessel containing Kr. Solution For monochromatic radiation the power is equal to the number of photons per second multiplied by the energy of each photon. Hence the number of X-ray photons per second is calculated from: QUESTION 2 An electroscope consists of a very thin piece of gold foil (known as gold leaf) fixed at the top to a rod of copper. A zinc plate is attached to the copper conductor, as shown in Figure 2. Figure 2: Schematic of a gold leaf electroscope. Charge can be transferred to the electroscope by bringing the zinc plate in contact with a charged object. The charge flows over the conducting copper and gold, and the gold leaf moves away from the copper rod as it is repelled by the charge on the copper conductor. (a) It is observed that when an initially negatively charged zinc plate is exposed to ultraviolet (UV) radiation the gold leaf collapses. Briefly explain this observation. Solution Ultraviolet (UV) radiation incident on the zinc plate ejects photoelectrons from the metal surface, which are repelled from the zinc plate by its negative charge. When negatively charged electrons are removed from the zinc plate, the remaining zinc atoms are positively charged. The zinc atoms are neutralised when electrons flow from the copper rod. As a consequence the repulsive force between the gold leaf and the copper rod decreases and the leaf collapses. This situation is illustrated schematically below. (b) If the zinc plate is charged in the same manner as in part (a), and the intensity of the UV light is increased what do you expect to happen? Solution The intensity of the UV light depends on the number of UV photons per second. Increasing the intensity of the UV light causes more photoelectrons per second to be ejected from the zinc plate, and consequently the gold leaf collapses more quickly. (c) X-ray photoelectron spectroscopy (XPS) uses photo-ionisation of electrons to analyse the surface composition of a material. Figure 3 shows a simplified energy level diagram for a sodium atom in NaCl. Figure 3: Energy level diagram for a sodium atom. If X-rays with energy 1.253 keV are used, calculate the kinetic energy of the sodium 2s photoelectron peak expected in an XPS spectrum. Solution The Einstein photoelectron equation is used to calculate the kinetic energy of the 2s photoelectron peak in the XPS spectrum, i.e., where hf is the energy of the incident X-ray photon (1.253 keV) and W is the photoionization energy (i.e., the energy required to remove an electron from the 2s level in sodium). From Figure 3 we note that for the 2s level in sodium, hence QUESTION 3 When white light between 400 nm and 700 nm is shone on atomic hydrogen a spectrum consisting of dark lines is observed (see Figure 4 ). Briefly discuss the origin of these lines in the spectrum of hydrogen. What determines the wavelengths of these spectral lines? 656nm 486nm 434nm 410nm Figure 4: Dark lines in the spectrum of atomic hydrogen between 400 nm and 700 nm. Solution Figure 4 shows the absorption spectrum of atomic hydrogen in the range 400 nm to 700 nm. When white light (having this range of wavelengths) is shone onto a gas of atomic hydrogen, photons with energy (wavelength) corresponding to differences in the energy levels of the hydrogen atom are absorbed, causing an electron to make an “upward” transition between quantized (discrete) energy levels. The wavelengths of the absorption lines are determined from: (1) where and Upon substituting This corresponds to the Balmer series of the hydrogen atom. and into Eq. (1) we obtain the wavelengths shown in Figure 4. _____________________ School of Physics & Astronomy PHS1022 Quantum Physics - Sample Problem Set 3 QUESTION 1 (a) When black and white photographic film is exposed to light, molecules of silver bromide (AgBr) contained in the photosensitive emulsion are dissociated (i.e., broken apart). The minimum energy required to dissociate a AgBr molecule is 0.68 eV. Calculate the maximum wavelength (in nm) beyond which this film will not record light. Solution Equating the dissociation energy of a AgBr molecule with the energy of the incident photon: Thus the maximum wavelength for dissociation is given by: (b) A 0.1 kg mass falls through a height of H = 1 m. If all the energy acquired in the fall were converted to optical photons (λ = 680 nm), how many photons would be emitted? Solution Equate the gravitational potential energy with the energy of the photons, according to: where N is the number of photons, each having energy hf. Hence we have: (c) Light form a laser diode (λ = 680 nm) is incident on an antimony oxide photocathode, whose work function is 2.0 eV. Clearly stating your reasons indicate if photoelectrons would be emitted from the surface of the photocathode. Solution The energy of a photon from the laser diode is: Since the energy of the incident photon is less than the work function for antimony oxide, no photoelectrons are emitted from the surface of the photocathode. QUESTION 2 A diffraction pattern is observed on a fluorescent screen when electrons are accelerated through a potential difference of 5 kV, and subsequently interact with a carbon thin film. Figure 1 shows the resulting diffraction pattern. Figure 1: Diffraction pattern observed when electrons interact with a thin film of carbon. (a) Calculate the de Broglie wavelength of electrons that give rise to the diffraction pattern in Figure 1. Solution The de Broglie wavelength is given by: Thus (b) Discuss briefly what happens to the diffraction pattern when the accelerating potential is reduced to 1 kV. Solution Since , if we reduce the accelerating potential from 5 kV to 1 kV, the de Broglie wavelength will increase by a factor of The separation of maxima in the diffraction pattern depends on wavelength according to: therefore is increased and the circular diffraction pattern expands. (c) The separation of the fringes in Figure 1 is approximately 1 cm. Estimate the distance between carbon atoms in the thin film. Clearly show how you arrived at your answer. Take the distance between the carbon film and the fluorescent screen to be D ~ 12 cm. Solution Assume that interference arises when two electron waves are scattered off adjacent carbon atoms in the thin film. We can model this on the basis of a Young’s double slit arrangement as shown in Figure 2. Screen xm ~ 1 cm Atom 2 Distance between d C-atoms Atom 1 D ~ 12 cm Figure 2: Interference between two electron waves scattering off adjacent carbon atoms. The locations of the maxima are given by: , where is the de Broglie wavelength calculated in Question 2 (i). Since we can use the following approximation: Hence we can write: This gives: is small A value of is in agreement with the expected interatomic distance for carbon atoms in a thin film. _________________________ School of Physics & Astronomy PHS1022 Quantum Physics - Sample Problem Set 4 QUESTION 1 (a) A laser diode produces optical radiation of wavelength 690 nm. Calculate the energy (in eV) of a single photon of light from the laser diode. Solution The energy of a single photon of light is given by: Converting to eV, where (b) gives: A 0.1 gram mass falls through a height of 1 cm. If all the energy acquired in the fall were converted to optical photons (8 = 690 nm), how many photons would be emitted? Solution Assuming all the gravitational potential energy is converted into photons, we have: where N is the number of photons and l is the height through which the mass falls. The number of photons is determined from: (c) Calculate the momentum of a single photon of light from the laser diode (8 =690 nm). Solution The momentum of the photon is given by: (d) Light form the laser diode is incident on an antimony oxide photocathode, whose work function is 2.0 eV. Clearly stating your reasons indicate if photoelectrons would be emitted from the surface of the photocathode. Solution The Einstein photoelectric equation determines the maximum kinetic energy of photoelectrons emitted from the surface of the photocathode, i.e., where W = 2.0 eV is the work function of antimony oxide. We note that Since the incident photon energy is less that the minimum amount of energy (i.e., the work function) needed to free an electron from the surface of the photocathode, there can be no photoemission. QUESTION 2 Bucky balls, or more correctly buckministerfullerene ( ), are large molecules consisting of sixty tightly bound carbon atoms in the shape of a soccer ball. Under certain circumstances a double slit interference pattern can be observed using bucky balls. (a) Hot molecules from an oven are collimated into a fine beam, which is incident on a double slit of separation, d, made from silicon nitride (SiN). A detector is used to record the number of molecules arriving at a point beyond the slit. The average speed of the molecules is 210 Calculate the de Broglie wavelength of the molecules. [Assume the mass of a bucky ball is: Solution The de Broglie wavelength of a molecule is given by: (b) In order to produce an observable interference pattern with bucky balls, the SiN double slit is chosen to have a slit separation of d = 150 nm. Calculate the spacing of the maxima in the interference pattern. Take the distance between the SiN double slit and the detector to be L = 1.0 m. Solution The maxima in the interference pattern are determined from where is the de Broglie wavelength of a bucky ball and m = 0,1,2,... is the order of the maximum. Since where we can use the approximation: is the distance to the mth maximum. Hence: and the spacing of the maxima (fringe spacing) in the interference pattern is given by: whence ________________________