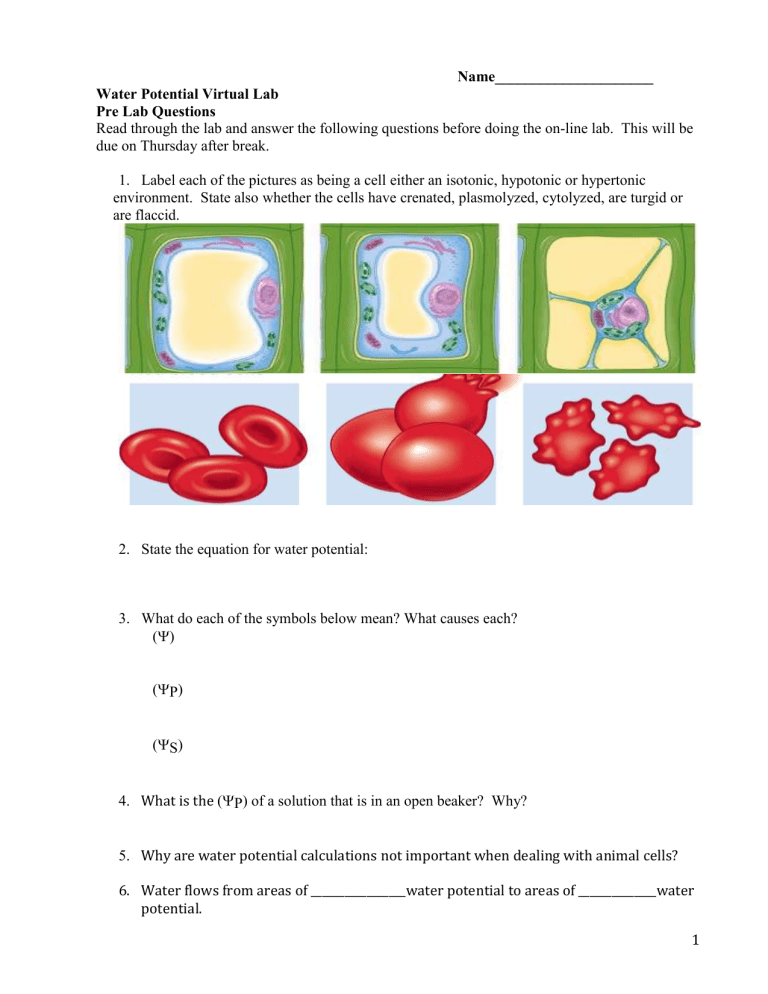
Name_____________________ Water Potential Virtual Lab Pre Lab Questions Read through the lab and answer the following questions before doing the on-line lab. This will be due on Thursday after break. 1. Label each of the pictures as being a cell either an isotonic, hypotonic or hypertonic environment. State also whether the cells have crenated, plasmolyzed, cytolyzed, are turgid or are flaccid. 2. State the equation for water potential: 3. What do each of the symbols below mean? What causes each? () (P) (S) 4. What is the (P) of a solution that is in an open beaker? Why? 5. Why are water potential calculations not important when dealing with animal cells? 6. Water flows from areas of _________________water potential to areas of ______________water potential. 1 7. Cell A has a S of -2.0 MPa and a P of +0.5 MPa and Cell B has S of -4.0 MPa and a P of +0.9 MPa Which way will water move when the two cells are placed against each other? Explain. 8. If a plant cell has a lower water potential than its surrounding environment and if pressure is equal to zero, is the cell hypertonic or hypotonic to its environment? Will the cell gain or lose water? Explain. Use the below diagram to answer the next 2 questions. 9. State the water potential for beaker A___________ and the water potential for beaker B_____________ 10. Which statement below is true? A. The beet core in beaker B will lose water to the surrounding environment. B. The beet core in beaker B would be more turgid than the beet core in beaker A. C. The beet core in beaker A is likely to gain so much water that its cells will rupture. D. The cells in beet core B are likely to undergo plasmolysis. E. The beet core in beaker A is at equilibrium with the surrounding water. 2 11. A wind borne pollen grain with an S of -3.0 MPa has dried out somewhat after blowing around in the wind. This has caused its turgor pressure, P to go to zero. It lands on a flower stigma whose cells have a S of - 3.0 MPa and P of +1 MPa. a. Which way will water flow? From the pollen grain to the sigma or the stigma to the pollen grain? ..............show how you deduced your answer: 3 Name________________________________ Water Potential Virtual Lab Targets addressed: Target XIV- Calculate and explain how solutions affect the water potential, pressure potential, and solute potential causing plant cells to plasmolyze, be turgid, or be flaccid. Target D--Design an experiment using different concentration of sucrose solutions to calculate the water potential of potato cells Introduction In this exercise you will use cores of potato tissue placed in different molar concentrations of sucrose in order to determine the water potential of potato cells. First, however, we will explore what is meant by the term "water potential." In animal cells, movement of H2O into and out of a cell is influenced by the relative concentration of solute on either side of the cell membrane. If water moves out of the cell, the cell will shrink or crenate and if water moves into the cell it will swell and may even burst or cytolyze. In plant cells, the presence of a rigid cell wall prevents cells from bursting as water enters the cells, but pressure eventually builds up inside the cell and the pressure affects the process of osmosis. To predict the direction in which water will diffuse through living plant tissues, a quantity known as water potential is used. Water potential is abbreviated by the Greek letter psi (). Water potential is the chemical potential of water (free energy per mole of water) and has two major components: osmotic potential (S), which is dependent on solute concentration, and pressure potential (P) which results from the exertion of pressure — either positive or negative (tension) on a solution. The formula for calculating water potential is: Water potential = Pressure potential + Osmotic (solute) potential () (P) (S) Water will always move from an area of higher water potential (higher free energy) to an area of lower water potential (lower free energy). Water potential, then, measures the tendency of water to leave one place in favor of another place. The water potential of pure water in a beaker open to the atmosphere is zero (= 0) because both the osmotic and pressure potentials are zero (S = 0; P = 0). An increase in positive pressure raises the pressure potential (makes more positive) and therefore raises water potential (). The addition of solute to water lowers the osmotic potential (makes S more negative) and 4 therefore lowers the water potential. Therefore, a solution at atmospheric pressure (P = 0) will always have a negative value for water potential due to the presence of solute in the solution. For instance, a 0.1M solution of sucrose at atmospheric pressure (P = 0) has an osmotic potential (S) of -2.3 bars due to the solute, and thus a water potential () of -2.3 bars. A bar is a metric measure of pressure, measured with a barometer, which is about the same as 1 atmosphere. When a solution, such as that inside a potato cell, is separated from pure water by a selectively permeable membrane, water will move (by osmosis) from the surrounding area where water potential is higher (= 0) into the cell where water potential is lower due to the presence of solutes (S is negative). We will assume, for purposes of explanation, that the solute is not diffusing (Figure 4.1). The movement of water into the cell causes the cell to swell and the cell membrane pushes against the cell wall to produce an increase in pressure (turgor). Eventually, enough positive turgor pressure builds up to oppose the more negative osmotic potential of the cell. This process will continue until the water potential of the cell equals the water potential of the pure water outside the cell ( of cell = of pure water = 0). At this point, a dynamic equilibrium is reached and NET water movement will cease. If you now add solute to the water outside the potato cells, the water potential of the solution surrounding the cells will decrease. It is possible to add just enough solute to the water so that the water potential outside the cell is the same as the water potential inside the cell. In this case, there will be no net movement of water. This does not mean, however, that the solute concentrations inside and outside the cell are equal, because water potential inside the cell results from the combination of both pressure (P) and solute concentration (S). If enough solute is added to the water outside the cells, water will leave the cells, moving from an area of higher water potential to an area of lower water potential. The loss of water from the cells will cause the cells to lose turgor. A continued loss of water will eventually cause the cell membrane to shrink away from the cell wall (plasmolysis). 5 Procedure 1. Go to the following website: http://www.neosci.com/demos/10-1041_Cell%20Processes/Labs_WaterPotential.swf 2. Since you have already read the objectives, safety, and materials sections for the pre-lab, go directly to Begin Activity. 3. Follow the directions under Steps 1, 2, and 3. Fill in the data table as you go. DO NOT use the notebook as it says to do in the steps. Title: The Effect of ________________________________ on _________________________________of ____________________________________ over______________________________ Data Analysis 1. Do the calculations necessary to complete the Data table. 2. On graph paper, create a graph showing the effect of the molarity of the surrounding solution on the % change in potato core mass. 3. Determine the water potential of the potato cores following the steps below. a. Determine the osmolarity of the sucrose solution in which the mass of the potato cores does not change. To do this, draw a line of best fit for your data points. The point at which the line of best fit crosses the x-axis represents the molar concentration of the sucrose solution with a water potential that is equal to the potato cells’ water potential. At the x-intercept concentration, there is no net gain or loss of water from the tissue. Write the Molar concentration of sucrose = _________________M (HINT: value = xintercept on graph) 6 b. The solute potential (S) of this solution can be calculated using the following formula: S solution = - iCRT i = ionization constant (for sucrose this is 1 because sucrose does not ionize in water) C = osmotic molar concentration (determined in step a above) R = pressure constant (handbook value R = 0.0831 liter bars/mole K T = temperature K (273 + °C of solution)—for this lab, temp is 22°C The units of measure will cancel as in the following example. A l.0M sugar solution at 22° C under standard atmospheric conditions: -(1)(1.0 mole/liter H2O)(0.083 1 liter bar / mole °K)(295 °K) = - 24.51 bars c. Having calculated the solute potential of the solution (S solution) and knowing that the pressure potential of the solution is zero (P solution = 0) allows you to calculate the water potential of the solution. The water potential of the solution ( solution) is equal to the osmotic potential of the solution (S solution). d. The water potential of the solution ( solution) at equilibrium (where your line of best fit crossed the x-axis) will be equal to the water potential of the potato cells ( potatoes). THE WATER POTENTIAL OF THE POTATO CORES IS____________ Analysis Questions: 1. The molar concentration of a sugar solution in an open beaker has been determined to be 0.3M. Calculate the solute potential at 27 degrees. Round your answer to the nearest hundredth. 2. A U-tube experiment is set up. The left side (side A) has 100mL of a 0.3M sucrose solution (S = -0.48MPa). The right side (side B) has 100mL of a 0.2M sucrose solution (S = -0.34MPa). A selectively permeable dialysis membrane that is impermeable to sucrose and permeable to water separates the two sides. A plunger applies a positive (push) pressure of 100MPa to the solution on Side B. Draw the initial U-tube experiment, calculate the water potential on each side of tube, and indicate the net direction of water movement. Explain why water moves in a particular direction. 7 3. At equilibrium, is the solute potential of the potato cells (S potato) the same as the solute potential of the solution (S solution)? Explain. 4. A potato dehydrates by sitting in a room. Is the water potential of the dehydrated potato higher or lower than original potato? Explain. 5. If sweet potato cores were used in this lab instead of regular potato cores, how would the results be different? Would you likely find the sweet potato cells to have a lower or higher water potential than the regular potato cores? Explain. 6. Explain why the water potential numbers are as they are on the diagram below. 8 9






