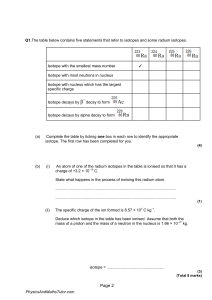
Across 1 Isotope of hydrogen with one neutron. (9) 6 When two atoms of different elements combine chemically, what does it produce? (7) 7 The lightest element. (8) 8 Elements that lend electrons in chemical reactions. (5) 9 A nuclear reaction in which the nucleus of two atoms combine to form one nucleus. (6) 11 Helium, neon, argon, or krypton. (5,3) 13 Where electrons reside in the atom. (5) 15 The most common isotope of carbon. (6,6) 16 The isotope of carbon that is used for dating things in archeology. (6,8) 17 Elements that borrow electrons in chemical reactions. (8) Down 2 An atom of an element with a different number of neutrons in the nucleus. (7) 3 Unstable. (11) 4 Positively charged particle in the nucleus. (6) 5 The center of an atom. (7) 9 A nuclear reaction in which an atom's nucleus splits to form two new atoms. (7) 10 Negatively charged particle. (8) 12 Isotope of hydrogen with two neutrons. (7) 14 Particle with no charge. (7)