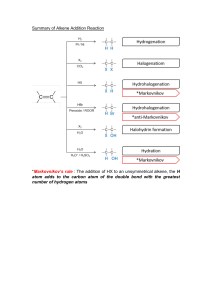
Lesson Eight Homework Reactions & Mechanisms of Alkenes (Part One) Name: Date: 1. When you have a carbocation in a reaction mechanism, why can the nucleophile add to different sides? (Note: this has to do with stereochemistry). 2. In your own words, explain what the term regiochemistry means as it applies to a reaction. 3. In your own words, explain what the term stereochemistry means as it applies to a reaction. 4. What is the official name of the “rule” that tells us that we get the more substituted product in an addition reaction? 5. Try to draw in the major product (note we mean the Markovnikov product) of the reaction shown. For the following questions, use the reaction below to answer them. 6. Give the major product (fill in the blank). 7. Fill in the arrows for the first step of the mechanism. (I helped a little bit here). 8. Why is the positive charge on carbon 2 and not carbon 1? Why isn’t it on carbon 3 or 4? 9. What is the last step of the mechanism? (Fill in the arrows in the diagram above). 10. There are two phrases that we normally look for in an addition reaction mechanism. One explains the regiochemistry and the second explains the stereochemistry. What are the two phrases that would accompany this mechanism? 11. How many steps are in the mechanism of the addition of water to a double bond, and how many involve the catalyst? 12. What is the first step of the mechanism of the acid catalyzed addition of water to a double bond? (hint: think about what the nucleophile is)…I have helped a little bit here by drawing in the protonated water. ☺ 13. Which carbon will have the charge in the carbocation intermediate from the step above? 14. Why is this the preferred carbocation? 15. What is the second step of the mechanism? Try to draw it in the space below. 16. The last step is the reformation of the catalyst/ deprotonation of the intermediate. I have filled in part of the steps, try to add the arrows…. 17. The next relatively common reaction is the elimination reaction. What are two common elimination reactions that we discussed in lecture today? 18. What does adjacent carbon mean in terms of chemistry? Why is this important to remember when trying to predict the product of an elimination reaction? 19. If you have a choice between hydrogens to remove, which is preferred? (I.e. if you have 2 bromo butane and add KOH, what is the preferred product, 1-butene or 2-butene? 20. What is the name of the “rule” that we use to predict the product of elimination reactions? 21. What are the products of the two reactions below? 22. There are reactions that give the less substituted (or anti-Markovnikov) product preferentially. In order to add HBr to an alkene to get the less substituted double bond, you need something called a radical initiator. Draw an example of a radical initiator in the space below. 23. What is the most stable type of radical? 24. What are the three “steps” of a radical mechanism? Give just the names in the space below. 25. What type of step is the one shown in each reaction below? a) b) a) b) 26. What is one advantage of using the oxymercuration addition of water to a double bond? 27. We just discussed the anti Markovnikov (radical addition) of HBr to an alkene. What is the reagent that can be used to add water to give the less substituted alcohol? 28. Why is BH3 electrophilic? 29. Since BH3 is electrophilic, it is approached by the nucleophilic double bond. Which carbocation shown below is preferred in the addition of BH3 to propene? Why? 30. After the carbocation is formed, the BH3 group is eventually replaced by the OH, which is why the OH group winds up on the less substituted carbon. Try to predict the product of the reaction shown below involving a hydroboration. 31. Give the products of each reaction shown below… Consider the following reaction conditions and answer the questions below… 32. Which reaction conditions below make the following transformation? _______________ 33. Which conditions give alcohols as products? _______________ 34. Which conditions will give anti Markovnikov addition to make an alkyl halide? _____________ 35. Which processes involve carbocations & give a Markovnikov addition? ______________ 36. Which conditions involve a ring? _____________ 37. Which processes give Markovnikov products? ___________ 38. Which conditions is a less acidic means of making a Markovnikov alcohol? ___________