Guide to Studying Reactions
advertisement
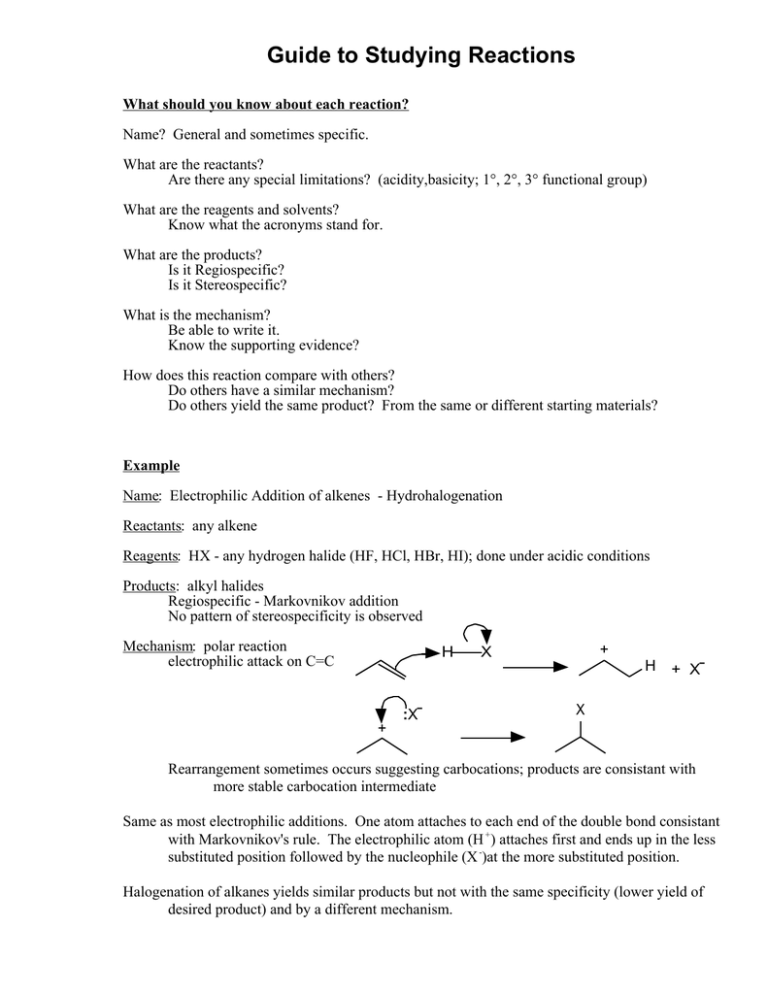
Guide to Studying Reactions What should you know about each reaction? Name? General and sometimes specific. What are the reactants? Are there any special limitations? (acidity,basicity; 1°, 2°, 3° functional group) What are the reagents and solvents? Know what the acronyms stand for. What are the products? Is it Regiospecific? Is it Stereospecific? What is the mechanism? Be able to write it. Know the supporting evidence? How does this reaction compare with others? Do others have a similar mechanism? Do others yield the same product? From the same or different starting materials? Example Name: Electrophilic Addition of alkenes - Hydrohalogenation Reactants: any alkene Reagents: HX - any hydrogen halide (HF, HCl, HBr, HI); done under acidic conditions Products: alkyl halides Regiospecific - Markovnikov addition No pattern of stereospecificity is observed Mechanism: polar reaction electrophilic attack on C=C H + :X- + X H + X- X Rearrangement sometimes occurs suggesting carbocations; products are consistant with more stable carbocation intermediate Same as most electrophilic additions. One atom attaches to each end of the double bond consistant with Markovnikov's rule. The electrophilic atom (H +) attaches first and ends up in the less substituted position followed by the nucleophile (X -)at the more substituted position. Halogenation of alkanes yields similar products but not with the same specificity (lower yield of desired product) and by a different mechanism.