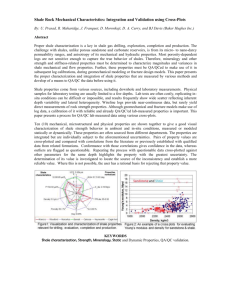
SPE 54356 Shale Stability: Drilling Fluid Interaction and Shale Strength Manohar Lal, SPE, BP Amoco Copyright 1999, Society of Petroleum Engineers Inc. This paper was prepared for presentation at the 1999 SPE Latin American and Caribbean Petroleum Engineering Conference held in Caracas, Venezuela, 21–23 April 1999. This paper was selected for presentation by an SPE Program Committee following review of information contained in an abstract submitted by the author(s). Contents of the paper, as presented, have not been reviewed by the Society of Petroleum Engineers and are subject to correction by the author(s). The material, as presented, does not necessarily reflect any position of the Society of Petroleum Engineers, its officers, or members. Papers presented at SPE meetings are subject to publication review by Editorial Committees of the Society of Petroleum Engineers. Electronic reproduction, distribution, or storage of any part of this paper for commercial purposes without the written consent of the Society of Petroleum Engineers is prohibited. Permission to reproduce in print is restricted to an abstract of not more than 300 words; illustrations may not be copied. The abstract must contain conspicuous acknowledgment of where and by whom the paper was presented. Write Librarian, SPE, P.O. Box 833836, Richardson, TX 75083-3836, U.S.A., fax 01-972-952-9435. Abstract This paper presents main results of a shale stability study, related to the understanding of shale/ fluid interaction mechanisms, and discusses shale strength correlation. The major shale/ fluid interaction mechanisms: Capillary, osmosis, hydraulic, swelling and pressure diffusion, and recent experimental results are discussed. Factors affecting the shale strength are discussed, and a sonic compressional velocity-log based correlation for strength is proposed. Recommendations for modeling and improving shale stability are described, based on the current understanding of shale stability. Introduction Shales make up over 75% of the drilled formations, and over 70% of the borehole problems are related to shale instability. The oil and gas industry still continues to fight borehole problems. The problems include hole collapse, tight hole, stuck pipe, poor hole cleaning, hole enlargement, plastic flow, fracturing, lost circulation, well control. Most of the drilling problems that drive up the drilling costs are related to wellbore stability. These problems are mainly caused by the imbalance created between the rock stress and strength when a hole is drilled. The stress-strength imbalance comes about as rock is removed from the hole, replaced with drilling fluid, and the drilled formations are exposed to drilling fluids.1 While drilling, shale becomes unstable when the effective state of the stress near the drilled hole exceeds the strength of the hole. A complicating factor that distinguishes shale from other rocks is its sensitivity to certain drilling constituents, particularly water. Shale stability is affected by properties of both shale (e.g. mineralogy, porosity) and of the drilling fluid contacting it (e.g. wettability, density, salinity and ionic concentration). The existence and creation of fissures, fractures and weak bedding planes can also destabilize shale as drilling fluid penetrates them. Drilling fluids can cause shale instability by altering pore pressure or effective stress-state and the shale strength through shale/fluid interaction. Shale stability is also a time-dependent problem in that changes in the stress-state and strength usually take place over a period of time. This requires better understanding of the mechanisms causing shale instability to select proper drilling fluid and prevent shale instability. The basic shale stability problem can be stated as follows: Shale with certain properties (including strength) normally lies buried at depth. It is subjected to in situ stresses and pore pressure, with equilibrium established between the stress and strength. When drilled, native shale is exposed suddenly to the altered stress environment and foreign drilling fluid. The balance between the stress and shale strength is disturbed due to the following reasons: • Stresses are altered at and near the bore-hole walls as shale is replaced by the drilling fluid (of certain density) in the hole. • Interaction of drilling fluid with shale alters its strength as well as pore pressure adjacent to the borehole wall. Shale strength normally decreases and pore pressure increases as fluid enters the shale. When the altered stresses exceed the strength, shale becomes unstable, causing various stability related problems. To prevent shale instability, one needs to restore the balance between the new stress and strength environment. Factors that influence the effective stress are wellbore pressure, shale pore pressure, far away in situ stresses, trajectory and hole angle, etc. The effective stress at any point on or near the borehole is generally described in terms of three principal components. A radial stress component that acts along the radius of the wellbore, hoop stress acting around the circumference of the wellbore (tangential), axial stress acting parallel to the well path, and additional shear stress components. To prevent shear failure, the shear stress -state, obtained from the difference between the stress components (hoop usually largest and radial stress - smallest), should not go above the shear strength failure envelope. To prevent tensile failure causing fracturing, hoop stress should not decrease to 2 the point that it becomes tensile and exceeds the tensile strength of the rock. The controllable parameters that influence the stress-state are drilling fluid, mud weight, well trajectory, and drilling/ tripping practices. For example, radial stress increases with mud weight (wellbore pressure) and hoop stress decreases with mud weight causing mechanical stability problem. The near wellbore pore pressure and strength are adversely affected by drilling fluid/shale interaction as shale is left exposed to drilling fluid (chemical stability problem). Mechanical stability problem can be prevented by restoring the stress-strength balance through adjustment of mud weight and effective circulation density (ECD) through drilling/ tripping practices, and trajectory control. The chemical stability problem, on the other hand, is time dependent unlike mechanical instability, which occurs as soon as we drill new formations. Chemical instability can be prevented through selection of proper drilling fluid, suitable mud additives to minimize/delay the fluid/shale interaction, and by reducing shale exposure time. Selection of proper mud with suitable additives can even generate fluid flow from shale into the wellbore, reducing near wellbore pore pressure and preventing shale strength reduction. Understanding Subsurface Shale The term shale is normally used for the entire class of finegrained sedimentary rocks that contain substantial amount of clay minerals. Sedimentologists find shale hard to work with since shale is fine grained, lacks well-known sedimentary structure (so useful in sandstones), and readily applicable tools The and models are not available to study shale.2 distinguishing features of shale (of interest to oil industry) are its clay content, low permeability (independent of porosity) due to poor pore connectivity through narrow pore throats (typical pore diameters range 3 nm-100 nm with largest number of pores having 10 nm diameter), and large difference in the coefficient of thermal expansion between water and the shale matrix constituents. To understand drilling fluid interaction with shale, one must start from basic properties of in situ shale (e.g. pre-existing water in shale, mineralogy, porosity), and then analyze the impact of changes in stress environment on the properties of shale. Several factors affect the properties of shale buried at various depths. The amount and type of minerals, particularly clay, in shale decide the affinity of shale for water. For example, shale with more smectite (surface area - 750 m2/gm) has more affinity for water (adsorbs more water) than illite (surface area - 80 m2/gm) or kaolinite (25 m2/gm). Three different types of water are found associated with clays, although each clay will not contain all of the types. Intercrystalline water is found in associated with the cations neutralizing the charge caused by elemental substitution. Osmotic water is present as an adsorbed surface layer associated with the charges on the clay. The swelling associated with this type of mechanism occur when M. LAL SPE 54356 sedimentary rocks are unloaded as occurs in drilling. Bound water is present in the clay molecule itself as structurally bonded hydrogen and hydroxyl groups which under extreme conditions, temperatures of 600-7000 C, separate from the clay to form water. The free water exists only within the pore space between the grains. The porosity of shale is normally defined as the percent of its total volume that water. This value is normally measured by drying a known volume of shale at elevated temperature. Porosity then is a measure of free water, osmotic water and to a lesser extent inter-crystalline water. Chemically bound water is not measured in this procedure. Properties of shale and drilling fluid/shale interaction are strongly influenced by the bound water and to a lesser extent by the free water. Some of water associated with clay can also be removed using pressure. The majority of the loosely held osmotic water can be removed with an overburden pressure of about 290 psi. In the inner-crystalline case, up to four layers of water may be found. The third and fourth layer can be removed with about 3900 psi. Approximately, 24000 psi is required for second mono-layer and according to various estimates,3-4 pressure over 50,000 psi is required to squeeze water in single monolayer of clay platelets. It requires temperatures in excess of 200o C to remove all bound water from clay. It is, therefore, doubtful that shale is ever completely void of water in typical drilling environment. Prior to drilling, the exact amount of bound and free water in shales buried at depth, however, depends on the past compaction history. Compaction of clay proceeds in three main stages.5 The clays are removed from land by water and deposited in quiescent locations. Clays, at their initial state of deposition and compaction, have both high porosity and permeability; pore fluids are in communication with the seawater above; sediments consisting of hydratable clay with absorbed water layers prevent direct physical grain-to-grain contact. At the time of deposition, mud water contents may be 70-90%. In the normal compaction process as clay/shale sediments are buried with pore water being expelled, porosity (sonic travel time) decreases. However, any disruption of this normal compaction and water expulsion process can lead to increase in both porosity (sonic travel time) and pore pressure. In the first stage of compaction, free pore-water, osmotic water and water inter-layers beyond two layers are squeezed out by the action of overburden. After a few thousand feet of burial, the shale retains only about 30% water by volume, of which 20-25% is bound interlayer water and 5-10% residual pore water. In the early stages compaction strongly depends on depth of burial, grain size (fine-grain clays have more porosity but compact easily), deposition rate (high rate results in excessive pore pressures and under-consolidation), clay mineralogy (monmorillonitic shale contain more water than illitic or kaolinitic shale), organic matter content, and geochemical factors (e.g. concentration of sodium salts affects porosity). SPE 54356 SHALE STABILITY: DRILLING FLUID INTERACTION AND SHALE STRENGTH In the second stage of compaction, pressure is relatively ineffective for dehydration that is now achieved by heating, removing another 10 to 15% of the water. The second stage begins at temperatures close to 100oC and diagenetic changes in clay mineralogy may also occur. The third and final stage of compaction and dehydration is also controlled by temperature but is very slow, requiring hundreds of years to reach completion and leaving only a few percent of water. To sum up, the properties of drilled shale formation, which are important for shale/fluid interaction and shale stability, are dictated by the past compaction history and the current in situ stresses and temperature. For example, affinity (thirst) for water of the shale at any depth depends on compaction/ loading history, in situ stresses, clay composition, and temperature. These factors also determine shale porosity, permeability and the amount of water squeezed out. Shale/Fluid Interaction Mechanisms Analysis of the available experimental data (O’Brien-GoinsSimpson Associates and University of Texas, Austin, Shell and Amoco sponsored Projects)6, clearly shows that the shale strength and the pore pressure near the bore-hole are indeed affected by fluid/shale interaction. Basic results confirmed by this analysis can be summarized as follows: • Activity imbalance causes fluid flow into/or out of shale • Different drilling fluids and additives affect the amount of fluid flow in or out of shale • Differential pressure or overbalance causes fluid flow into shale • Fluid flow into shale results in swelling pressure • The moisture content affects shale strength. Moisture content relates to sonic velocity. The instability and shale/fluid interaction mechanisms, coming into play as drilling fluid contacts the shale formation, can be summarized as follows.7-8 1. Mechanical stress changes as the drilling fluid of certain density replaces shale in the hole. Mechanical stability problem caused by various factors is fairly well understood, and stability analysis tools are available.8 2. Fractured shale - Fluid penetration into fissures and fractures and weak bedding planes 3. Capillary pressure, pC, as drilling fluid contacts native pore fluid at narrow pore throat interface. 4. Osmosis (and ionic diffusion) occurring between drilling fluid and shale native pore fluid (with different water activities/ ion concentrations) across a semi-permeable membrane (with certain membrane efficiency) due to osmotic pressure (or chemical potential), PM. 5. Hydraulic (Advection), ph, causing fluid transport under net hydraulic pressure gradient because of the hydraulic gradient. 6. Swelling/Hydration pressure, ps, caused by interaction of moisture with clay-size charged particles. 7. Pressure diffusion and pressure changes near the wellbore (with time) as drilling fluid compresses the pore fluid and 8. 3 diffuses a pressure front into the formation. Fluid penetration in fractured shale and weak bedding planes can play a dominant role in shale instability, as large block of fractured shale fall into the hole. Several papers have been written on this phenomenon.9 In Norway Valhall field, this phenomenon is suspected to be one of the major causes of shale instability. Preventive measures include use of effective sealing agents for fractures, e.g. graded CaCO3, high viscosity for low shear rates, and lower ECD. Capillary phenomenon also is now fairly well understood, and an interesting exposition is given in a recent paper.10 Increasing the capillary pressure for water-wet shale has been successfully exploited to prevent invasion of drilling fluid into shale through use of oil base and synthetic mud using esters, poly-alpha-olefin and other organic low-polar fluids for drilling shale. The capillary pressure is given by pC = 2γ cosθ/r .................................................................(1) where, γ is interfacial tension, θ is contact angle between the drilling fluid and native pore fluid interface, and r is the pore radius. When drilling water-wet shale with oil base mud, the capillary pressure developed at oil/pore-water contact is large because of the large interfacial tension and extremely small shale pore radius. It prevents entry of the oil into shale since the hydraulic overbalance pressure, ph (=Pw-po), is lower than the capillary threshold pressure, pC. In such a case, advection (and pressure diffusion) cannot occur. However, osmosis and ionic diffusion phenomena can still occur under favorable conditions. Capillary pressure thus modifies ph and the net hydraulic driving pressure ph‘is given as follows: 0 < pC < ph ph ′ = p h − p C , ..................................(2) p h ′ = 0, pC > p h Capillary pressures for low permeability water-wet shales can be very high (about 15 MPa for average pore throat radius of 10nm). This is one of the key factors in successful use of oil base muds or synthetic muds using esters, poly-alpha-olefin and other organic low-polar fluids. Osmotically induced hydraulic pressure or differential chemical potential, PM, developed across a semi-permeable membrane is given by 10-12, PM = - ηPπ = - η (RT/V)ln(Ash/Am)..................................(3) where, η is membrane efficiency, Pπ is the theoretical maximum osmotic pressure for ideal membrane (η=1), R is the gas constant, T is the absolute temperature, V is the molar 4 M. LAL volume of liquid, and Am, Ash are the water activities of mud and shale pore fluid, respectively. Various expressions have been obtained for the membrane efficiency in terms of parameters that are difficult to measure. Two such expression are:11 η = 1 − ( a − rs ) 2 / ( a − rw ) 2 .............................................(4) η = 1 − νs / ν w where, ‘a’ is pore radius, rs is solute radius, rw is water molecule radius, and νs and νw are the velocities of solute and water, respectively. From non-equilibrium thermodynamics principles, assuming slow process near equilibrium and single nonelectrolyte solute, the linear relations between the pressure and flow can be written as 11-12 Jv ∆x = Lpph – Lp η Pπ......................................................(5) Js ∆x = Cs(1- η)Jv+ ωPπ....................................................(6) Jv =JwVw + JsVs................................................................(7) where Eqn. 5 simply states that the fluid flux Jv into shale is the superposition of fluxes due to hydraulic pressure gradient ph (advection) and due to osmotically induced pressure, PM (=η Pπ), related through the hydraulic permeability coefficient Lp. The coefficient Lp is related to the shale permeability, k, and filtrate viscosity, µ, as Lp= k/µ. Eq. 6 describes the net salt flux Js into the shale. Eq. 7 simply expresses the mass balance in terms of the water and salt flux and partial molar volumes of these components. Note that for perfect membrane, η =1, since only water can flow across the membrane, Js=0 and thus ω =0. Hydraulic (Advection), ph, is implicitly included in Eq. 5. If the test fluid is the same as shale pore fluid (which implies equal activity and Pπ =0 - no osmosis), Eq. 5 reduces to the familiar Darcy’s law which gives volume flow as: Jv ∆x = Lp ph...................................................................(8) where as Lp= k/µ.; k denotes shale permeability and µ denotes viscosity. Such an experiment was performed by van Oort to characterize the permeability of shale and estimate Lp. As stated earlier, a recent study on osmotic and hydraulic effects was conducted at O’Brien-Goins-Simpson & Associates, Inc. as part of the work sponsored by the Gas Research Institute (GRI). General conclusions from the study can be summarized as follows: • Increased hydraulic potential can increase the amount of transport of water into shales and reduce rock strength SPE 54356 (with exposure time). Increasing the mud weight may thus worsen a stability problem (over time) rather than curing it. • In hydrocarbon-based fluids, water transport into shales may be controlled through the activity of the internal phase relative to the shale. • Water-based fluids require a much lower activity than the shale to control water transport. Even then the effective strength may be reduced. Swelling pressure and swelling behavior of shales is directly related to the type and amount of clay minerals in a given shale. Two types of swelling observed in clays are: a) Innercrystalline swelling (IS) - caused by hydration of the exchangeable cations of the dry clay b) Osmotic swelling (OS) - caused by large difference in the ionic concentrations close to the clay surfaces and in the pore water. It may be noted that the osmosis, discussed earlier, was concerned with ionic concentration or water activity differences between the drilling fluid and the pore water. The swelling stress due to inner-crystalline swelling (IS) can be very large (approximately up to 58000 psi for the formation of first water layer, up to 16000 psi for second, and up to 4000 for the third and fourth layers for pure montmorillonite in the Wyoming bentonite). The swelling stresses resulting from osmotic swelling (OS), on the other hand, are relatively small and usually do not exceed 300 psi.13 Complete understanding of physico-chemical reactions between clays and water requires a detail discussion of the structure of compacted clay, namely the arrangement of clay particles at the atomic level and the electrical forces between the adjacent particles. The electrical forces act only near the particle surface and mostly result from discontinuity at or near the surface, they become particularly significant and dominate the mass forces (such particles are called colloidal - 1micron 1 millimicron (10A) size range) for clays since they have large surface area per unit mass. Several excellent papers13 are available which attempt to describe various aspects of this complex phenomenon. A simple explanation, which may suffice for this report, is given as follows. First the electrical forces: van der Waals forces (secondary valence forces) between units of clay arise from electrical moments existing within units, which are similar to force acting between two short bar magnets. Since there are more attractive positions than repulsive, the net effect of such forces is attraction. These forces exist in clays because of the nonsymmetrical distribution of electrons in the silicate crystals, which act as a large number of dipoles. They can attract other dipoles like water molecules, which are permanent dipoles due to nonsymmetrical configuration of the water molecules and position of the atoms in the molecule. The hydrogen bond linkage between water molecules occurs as each hydrogen atom, attracted by the oxygen in neighboring water molecule, links its water molecule to others. Furthermore, clay particles carry a net negative charge SPE 54356 SHALE STABILITY: DRILLING FLUID INTERACTION AND SHALE STRENGTH (mainly caused by isomorphous substitution - e.g. substitution of bivalent Mg for trivalent Al). This net negative charge is balanced by exchangeable cations, clustered at the clay surface to neutralize the particles. When the dry clay particle is placed in water the cations swarm around the clay surface particles, forming a double layer with certain electrical potential that vary with characteristics of the dispersion medium (according to Gouy-Chapman theory). Because of the net negative charge, adjacent clay particles repel each other as they approach each other close enough for the double layers to overlap. Combining this with the secondary valence attractive forces, equations for total potential energy have been developed. If the total potential energy reduces when adjacent particles approach each other, they flocculate (form aggregates), but if it increases they disperse or move apart. Confining discussion to clay-water reaction, the clay-water attractive force consists of two main components: attraction of dipolar water to the electrically charged clay particles and attraction of the dipolar water to the cation in the double layer - the cations in turn are attracted to the clay. Based on relative magnitude of force between water and clay (large near but becoming weak away from the colloidal surface), water can be categorized into three types: adsorbed - strongly held by clay; double layer - all the water attracted to clay anywhere in the double layer; and free water which is not attracted to clay at all. For illustration, let us look at two clay minerals: montmorollinite and kaolinite. For both minerals, the force required to pull the adsorbed water off the mineral surface is extremely high (varying from 100 for outside to 10,000 atmospheres for the closest molecules). The adsorbed and double layer water on kaolinite are thicker than on montmorillonite because of the high charge density on kaolinite (about twice). However, the amount of adsorbed water expressed, as a percentage of mineral weight is much greater on montmorillonite since its specific surface is greater. The controversy regarding particle mineral-to-mineral contact in clay is not yet settled. According to one concept, cohesion in a natural clay is due to “water bonds”20. Compaction and any other stress changes on clay minerals in shale also affect the clay structure and thus water-clay interaction. Swelling experiments indicate that the swelling follows a diffusion type of law, and the cumulative water flux into the shale, Q, time t, sorptivity S, the change in equilibrium void ratio (liquid to solid volume ratio) ∆e, and diffusivity, D, are related as follows 14: Q = S.t 0.5 S = ∆e.(2D)0.5...................................................................(9) The linear dependence of S on the change in equilibrium void ratio on swelling implied in the above equation is observed experimentally. Diffusivity for Pierre shale inferred from experiments is about 9x10-10 m2s-1 at 20o C. D depends 5 on the nature of bound cations, and commonly used polymers appear to have little effect on its value. The low values of D for typical shales may also explain why many shales become unstable after several weeks. Exchange of the natural bound cation (Na+, Ca2+, Mg2+) by K+ can lead to a clay fraction with lower swelling tendency. The experimental results, however, seem to indicate that a large fraction of the clay cations must be replaced before this effect is significant, and the action of KCl is largely osmotic since on the time scale of the swelling experiments little ion exchange with clay has taken place. Atmospheric swelling and saturation experiments on Speeton shale core were also sponsored by the GRI at the University of Texas, Austin. An interesting conclusion from this study was that the activities of the shale-fluid system, nature of the ions, ionic concentration, hydrated ionic diameter and valency of the generated cations influence the ultimate swelling response of shale. The results of the study indicate that water activity differential is not the only mechanism of water transportation into shale matrix under atmospheric conditions. The Electro-chemical forces associated with negatively charged clay surfaces and ionic exchange may transport water into shale matrix even in the presence of low activity salt solutions. In summary, swelling phenomenon in shales can be explained in terms of clay-water reaction, and water has significant effect on the properties of clays. Pressure diffusion phenomenon concerns the pressure change with time near the wellbore as the drilling fluid at wellbore pressure, Pw, in conjunction with the osmotic pressure, PM, etc. suddenly contacts and compresses the pore fluid at the wellbore wall (which was at pressure, po, before drilling). The pressure away from the wall varies with time until a steady-state pressure distribution between near and faraway pore pressure is established. This pressure diffusion can be compared in a way to the pressure surge when a pipe suddenly moves in a wellbore compressing the drilling fluid, which is analyzed as transient wave propagation instead of diffusion phenomenon. Various expressions and numerical simulations for pressure diffusion have been used to study this phenomenon 15-16. The basic point of these studies can be illustrated with the help of Fig. 1. If a drilling fluid cannot penetrate shale at all (e.g. perfect oil base mud for a given shale), the pore pressure near the wellbore wall is the virgin pore pressure po (ignoring the effect of stress changes) at the time drilling fluid comes in contact with shale (t=0) and remains the same for t>0. However, when the mud is such that it interacts with shale, the drilling fluid at wellbore pressure Pw will diffuse through shale. The pressure near the wall in the pores will increase from po with time. How fast this pore pressure in the vicinity of the borehole increases depends upon the permeability of shale, its elastic properties and other boundary conditions. In general, lower the permeability, more time it takes for pressure to increase and tend to equalize with Pw, thus losing pressure support for the formation. Depending on permeability, it may take anywhere 6 M. LAL from a few hours to a number of days before the pressure near the wellbore approaches the wellbore pressure, losing pressure support, reducing effective stresses and bringing the rock to unstable situation. This could be an explanation for the delayed failure of exposed shale sections, often experienced in the field. Shale Strength Correlation For stability analysis, an important input parameter needed is the formation strength, which is usually characterized by cohesive strength S0, and friction angle φ . These parameters are traditionally determined from different rock mechanical core tests based on a number of different core plugs from the same depth. The test results from several plugs are then combined to provide these strength properties from this depth. Regarding rock strength, it is far easier to make rock grains slide past one another than it is to crush them. Consequently, when rocks fail in compression, they are actually failing in shear, as a result of inter-granular slip. Their resistance to shear, i.e. shear strength, is due to a combination of cohesion and friction between the rock grains. The amount of cohesion is represented by a parameter known as the cohesive strength S0, while inter-granular friction is defined by the internal friction angle φ. For a layer of rock subjected to an effective compressive stress σ and a shear stress τ, the shear failure criterion can be written simply as: τ = S0 + σ tan φ..............................................................(10) Where, the effective compressive stress σ is related to the total compressive stress s and pore pressure p as follows: σ = s - p..........................................................................(11) For complex stress states, such as exist at the wall of a wellbore, a number of different failure criteria have been proposed for generalizing Eq. 10. However, all the criteria are related to the parameters S0 and φ. For example, the Mohr-Coulomb6,8 shear failure criterion can be written as: (σ1+σ3) (σ1-σ3) = So cosφ + sinφ..................................(12) 2 2 Where, (σ1-σ3)/2 is the Mohr Coulomb shear strength parameter, and (σ1+σ3)/2 is the average effective stress, and σ1, σ3 are the maximum and minimum effective compressive stresses, respectively. The Drucker-Prager Criterion is defined in terms of the two generalized stresses, the mean effective stress: I1 = (σ1 + σ2 + σ3 )/3......................................................(13) SPE 54356 and an equivalent shear stress parameter √J2, where √J2 = ((σ1-σ2)2+(σ1-σ3)2+(σ2-σ3)2)/6 .........................(14) and σ2 is the intermediate effective compressive stress. The Drucker-Prager Criterion is8: √J2 = m I1 + τo ..............................................................(15) where, in terms of So and φ: m = 2√3 sin φ /(3-sin φ)..............................................(16a) τo = 2√3 So cos φ /(3-sin φ)...............................................(16b) S0 and φ can be determined from laboratory triaxial strength tests, in which cylindrical samples of rock are first subjected to a hydrostatic confining pressure, and an axial load is then applied until the rock fails. These tests are performed at several different confining pressures, and the results are plotted as a series of Mohr’s circles, as shown in Fig. 2. For linear failure criterion, the line that envelops the family of circles has a slope equal to tanφ, and an intercept equal to S0. Intuitively, we know that rock strength tends to increase with compaction. Consequently, this suggests that S0 and φ can be tied to compaction-dependent wire-line measurements, such as porosity, density, and sonic velocity. However, determining S0 and φ on a foot-by-foot basis presents more of a challenge. It clearly is not feasible to do this with laboratory strength tests. As an alternative, it is desirable to develop relationships for computing S0 and φ from wire-line data. Therefore, rock strength correlation actually refers to relation with wire-line log data for determining the cohesive strength and friction angle. A more fundamental look at shale physics was taken to gain better insight into which factors need to be included in strength correlation. Three factors were considered6: • clay mineralogy • clay content • compaction. The main conclusion from the study, based on several external and internal data sources, appears to be as follows. Under in situ stress and native pore fluid salinity conditions, clay mineralogy and contents are of secondary importance regarding their effect on shale strength. The degree of compaction (characterized by water content, porosity, sonic velocity, etc.) appears to be the dominant factor. Thus, strength can be tied to any of the following related parameters: • water content • porosity • sonic velocity SPE 54356 SHALE STABILITY: DRILLING FLUID INTERACTION AND SHALE STRENGTH • density The shale strength correlations, developed by the author, were tied only to compressional sonic velocity in shales. The relations were developed using an extensive shale database. The following relations for friction angle, φ (degrees) , and cohesive strength, So (MPa), were developed as a function of compressional sonic velocity Vp (km/sec): sin φ = (Vp - 1)/( Vp + 1) So = 5(Vp-1)/√Vp , or = 10 tanφ.......................................................................(17) Fig. 3 plots the velocity based strength estimates computed from both laboratory measured and sonic log-derived velocities (reported for different core depths in North Sea), along with the measured strength data. Both estimates are fairly good, which show that this correlation is applicable with sonic log derived velocities. The sonic correlation was also found to be fairly satisfactory for formations other than shale. The estimated cohesive strength and friction angle parameter represent local values at the in situ stress conditions reflected in the sonic log measurements. For sands, Gassman Correction for gas needs to be applied, or nearby shale points needs to be picked as in pore pressure estimation. The impact of clay mineralogy and contents on strength (and stability) can become quite significant while drilling, when a foreign drilling fluid contacts in situ smectitic shale and alters the salinity of native pore fluid through shale/fluid interaction. Smectitic shales have a lower tolerance to drilling fluid invasion, and will tend to fail easier than formations in which kaolinite and/or illite are the only clay types present. The effect of clay mineralogy on strength can be important if the drilling process severely disturbs a formation from its natural state. In those cases, as discussed below, smectitic formations will be more susceptible to failure. The strength of all geologic materials depends upon the effective confining. Therefore, if shale/drilling fluid interaction raises the pore pressure in the near wellbore region, the drop in effective confining pressure will make the hole more susceptible to failure. However, with smectites, drilling can introduce two additional destabilizing effects. As discussed earlier, confining pressures in the vicinity of 500 psi are necessary to keep liquid water from getting in between smectite platelets. The two-to-three layers of water that remain are more competent than liquid water, and appear to allow smectite platelets to act like thicker, stronger particles. This effect can be reduced or lost if the effective confining pressure drops to values low enough to permit liquid water to penetrate between the platelets. Salinity can also cause smectite platelets to behave like thicker particles. However, as reported earlier this effect is not 7 permanent. A drop in salinity can cause a loss in strength. Therefore, a high activity (low salinity) mud could cause a significant drop in the strength of smectitic formations. To summarize, smectitic formations are highly susceptible to the effects of drilling fluid/shale interaction. Fluid invasion not only reduces friction and interlocking between smectite grains, it can also reduce the competency of the grains themselves. The impact of clay mineralogy on strength (and stability) can thus become quite significant on drilling, when a foreign drilling fluid contacts in situ smectitic shale and alters the salinity of native pore fluid through shale/fluid interaction. Smectitic shales have a lower tolerance to drilling fluid invasion, and will tend to fail easier than formations in which kaolinite and/or illite are the only clay types present. Finally, the effects of drilling fluid/shale interaction must be kept in mind when using offset well log data. Wireline log readings may not reflect true in situ pore pressure and rock properties if the near wellbore region has been invaded by the drilling fluid and undergone hydration. Hydration raises the local pore pressure and weakens the rock. Improving Shale Stability Thus far, we have seen that there are several mechanisms which cause or affect shale/fluid interaction. There is an intense effort under way in the oil industry to get a better understanding of each of these mechanisms. The stakes are high in that understanding and quantification of each of these phenomena is critical for designing benign drilling fluids which would stabilize shales. Rapid progress is being made and more results will become available in the near future. The current understanding of various mechanisms responsible for shale/fluid interaction indicate certain basic principles for improving shale stability. Based on current understanding of various shale/fluid interaction mechanisms, we can discuss some general principles for improving shale stability. The main objective to improve shale stability is to prevent, minimize, delay or use to our advantage the interaction of the drilling fluid with shale. As our understanding of the various interaction mechanisms improves, so will the mud systems designed to improve shale stability. We can list the following means of improving shale stability corresponding to various mechanisms contributing to shale/fluid interaction: • For fractured shale stability, use effective sealing agents, thixotropic drilling fluid (high viscosity for low shear rates), and lower mud weight /ECD. This would minimize fluid penetration into fractures. • Increase the capillary pressure, pC(>ph’) to prevent fluid entry into shale pore throats. Eq. 1 suggests that increasing interfacial tension and contact angle θ can increase the capillary pressure for given shale pore throat radii. Increasing capillary pressure through γ and θ for water-wet shales has been successfully exploited through use of oil base muds or synthetic muds using esters, poly- 8 • M. LAL alpha-olefin and other organic low-polar fluids. Reduce the total net driving force (pressure) for shale/fluid interaction. The net effective driving force (pressure) at t=0+ for pC<ph(=Pw-po) can be written as: ph‘= Pw - po - pC+ PM......................................................(18) which brings about the changes with time in the near wellbore pore pressure through pressure diffusion or transmittal and fluid transport into (or out of) the shale. The near wellbore pore pressure, pn, can be expressed in terms of the original virgin pore pressure, po, and time changes, δp(t), as: pn = po + δp(t)............................................................... (19) to minimize δp(t), we need to minimize ph′ , which can be accomplished by increasing capillary pressure pc, as discussed above, or making osmotic pressure PM equal to (or less than) zero by matching (or making drilling fluid activity, Am, lower than) shale water activity, Ash. If the activity of the mud is higher than that of the shale, we need to reduce membrane efficiency as much as possible. However, when drilling fluid activity is made lower than shales, resulting in negative osmotic pressure and causing pore fluid to flow out of shale into the wellbore, the membrane efficiency needs to be increased. Reduction of drilling fluid activity, Am, is at the heart of most inhibitive muds 14. This reduction is brought about by adding electrolytes: seawater bentonite muds, saturated saltpolymer (xanthan, guar), KCl or NaCl-polymer (PHPA, xanthan), fresh water calcium treated muds (lime, gypsum). A new type of drilling fluid based on a substituted sugar, methyl gluocide, is currently being looked at because of its ability to form low activity muds with high membrane efficiency. The dispersed water phase in oil base muds is treated to adjust the activity, usually with CaCl2, to make activity Am<Ash. • Slow down the rate of fluid transport and pressure diffusion rate. It is difficult to balance water activity of shale with mud exactly everywhere in a well because shale activity is not known and varies with depth and mineralogy. We can, nevertheless, control parameters that enable us to reduce the fluid transport and pressure diffusion rates by increasing the fluid viscosity and reducing the permeability of shales. Regarding the viscosity increase, the problem is to find solutes that increase the fluid viscosity significantly and yet can pass through the narrow shale pore space to maintain high viscosity. Most mud polymers are too large to enter shale but some low molecular weight polymers might achieve the desired results. As regards reducing permeability, one solution is to form permeability barrier at shale surface or within micro-fractures. Oil base mud achieves this as water is made to diffuse through continuous oil phase to reach the shale. Silicate and ALPLEX muds, for example, attempt to reduce the permeability. SPE 54356 Cationic polymers, which are strongly adsorbing, can also act in the same way. In the extreme, shale formation could be completely isolated by creating an impermeable hydrophobic seal, using asphaltine derivatives like gilsonite. Use of charged emulsifiers for binding the oil droplets of oil-in-water emulsions to the clay surface and organophilic clays in oil base muds could achieve similar results. Although changing the clay cation with less hydratable K+ or Ca2+ can reduce intrinsic swelling, these ions lead to more open structure and thus increase permeability. Work is currently underway to formulate drilling fluids containing cesium, Ce+ for stabilizing the shale. While this fluid would be very expensive to formulate, increased stability and rate of penetration could compensate for this cost. • Preserve mechanical integrity of the shale cuttings. As damage control, certain measures can be taken to limit the dispersion of cuttings or spallings by binding the clay particles together, if shale failure or erosion is initiated. Polymers that can reduce shale disintegration must adsorb onto clay platelet surface and have high enough energy to resists mechanical or hydraulic forces pulling them apart. PHPA and strongly adsorbing cationic polymers and components like polyglycerol can limit the dispersion of shale cuttings or spallings in the well. To achieve similar results within the shale formation, polymer must be able to diffuse into the bulk shale, requiring short flexible chains. Future work on shale stability and understanding shale/fluid interaction is bound to lead to better means to stabilize shales and design of environmentally acceptable effective mud systems. As new additives for drilling fluids are studied to stabilize shales, major challenge would be to make them compatible with preserving other desirable mud properties such as, rheology, drilled solids compatibility and drilling rates. Finally, even if we could design the best mud system for shale formations, continuous monitoring and control of drilling muds are critical elements for successful drilling. The mud composition continually changes as it circulates and interacts with formations and drilled solids. Unless concentrations of various mud additives are continually monitored (as opposed to the current practice of periodically monitoring just rheological and simple properties) and maintained, the desired results could not be achieved. The development and introduction of improved monitoring techniques for chemical measurements should proceed simultaneously with the development of more effective mud systems for shale stability, based on improved understanding of shale/fluid interaction. Conclusions The above discussion gives an indication of the experimental activity and progress in shale stability projects, sponsored by oil and gas industry. However, understanding of the shale/fluid interaction mechanisms is not yet complete to effectively control the shale instability problem. The ongoing developments and data from numerous active industry SPE 54356 SHALE STABILITY: DRILLING FLUID INTERACTION AND SHALE STRENGTH sponsored shale stability projects, hopefully, will provide answers to the remaining questions. Then only, one can develop models that can quantify the impact of shale/fluid interaction on the stress- strength of the shale and the timedependent effects. In view of the shale instability costs, it is imperative to understand shale behavior and its interaction with different fluids. Completely satisfactory answers to questions such as: which drilling fluid to use for drilling a particular shale, or how long can we keep the hole exposed to a particular fluid without causing shale instability, can be given only after such an understanding. The quantification of the impact of fluid invasion on effective stresses and shale strength near the wellbore is critical for shale stability analysis models. Simple and realistic shale testing procedures and shale/ fluid interaction testing procedures are required in order to achieve practical assessments of wellbore instability risks. Efforts to develop predictive models and to develop more effective fluids for drilling shales, based on improved understanding of shale/fluid interaction mechanisms must continue. Vp = γ = θ = η= νs = νw = µ = ∆e = φ = σ = τ = σ1 = σ2 = σ3 = Nomenclature Am = water activities of mud Ash = water activity of shale D = diffusivity Jv = fluid flux Js = solute flux k = shale permeability Lp = hydraulic permeability coefficient PM = observed osmotic pressure Pw = wellbore pressure Pπ = theoretical osmotic p = pore pressure pC = capillary pressure ph = hydraulic pressure ph′ = net hydraulic pressure ps = swelling pressure Q = cumulative water flux R = gas constant r = pore radius rs = solute radius rw = water molecule radius S = sorptivity S0 = cohesive strength s = total compressive stress T = absolute temperature t = time V = molar volume Bowers, help and wellbore are also compressional sonic velocity surface tension contact angle membrane efficiency solute velocity water velocity filtrate viscosity liquid to solid volume ratio internal friction angle effective compressive stress shear stress effective maximum principal stress effective intermediate principal stress effective minimum principle stresses References 1. 2. 3. Acknowledgements The author would like to thank Glenn Tron Kristiansen and Calvin Deem for their valuable discussions. Their contributions and help in several stability projects, related to shale/fluid interaction, gratefully acknowledged. 9 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. Lal et al. Amoco Wellbore Stability Team, “Amoco Wellbore Stability Drilling Handbook,” 1996. Potter, E. P, Maynard, J. B., Pryer, W. A.: Sedimentology of Shale, Springer-Verlag, N. Y., 1984. Mody, F. K. and Hale, A. H.: “A borehole Stability Model to Couple the Mechanics and Chemistry of Drilling Fluid Shale Interaction, SPE/IADC 25728, Proc. 1993 SPE/IADC Drilling Conference, Amsterdam, Feb. 23-25, 1993. van Olphin, H.: “Compaction of Clay Sediments in the Range of Molecular Particle Distances, Clays and Clay Minerals,” Proc. Eleventh National Conference on Clays and Clay Minerals, Ottawa, Ontario, Canada, August 13-17 (1962). Burst, J. F.: “Diagensis of Gulf Coast Clayey Sediments and Its Possible Relation to Petroleum Migration,” Amer. Assn. Pet. Geol. Bull., 53, pp.73-93, 1969. Lal, M., Kristiansen, T., Deem, C. and Bowers, G. “Shale Stability: Drilling Fluid/ Shale Interaction Study and Shale Strength Correlations,” Amoco Report F96-P-99, 963480010 ART Lal, M. and Deem, C., “Shale Stability: Drilling Fluid/Shale Interaction - State of the Art Report,” F95-P-117, 953420005-TUL, December 7, 1995. Amoco Wellbore Stability Team, “State of the Art in Wellbore Stability,” F94-P-60, June 20, 1994. Santarelli, F., Dardeau, C., and Zurdo, C.: “Drilling through highly fractured formations: A problem, a Model, and a Cure,” paper SPE 24592 presented at the 1992 Annual Technical Conference & Exhibition, Washington, D.C., Oct. 4-7 Forsans, T. M. and Schmitt, L., “Capillary Forces: The Neglected Factor in shale Instability Studies?” SPE Paper 28058 presented at the 1994 SPE/ISRM Rock Mechanics in Petroleum Engineering Conference, Delft, Aug. 29-31, 1994 Oort, van E., Hale, A. H., Mody, F. K. and Roy, S.: “Critical Parameters in Modelling the Chemical Aspects of Borehole Stability in Shales and in designing Improved Water-Based Shale Drilling Fluids,” SPE 28309 paper presented at the SPE Annual Conference, New Orleans, Sept. 26-28, 1994. Fritz, S. J. “Ideality of Clay Membranes in Osmotic Processes: A review,”: Clays and Clay Minerals, Vol. 34(2), 1986, pp. 214223. Madsen, F. T. and Muller, V.: “The Swelling Behavior of Clays,” Applied Clay Science, Vol. 4, 1989, pp. 143-156. Bailey, L., Denis, J. H., Maitland, G. C.: “Drilling Fluids and 10 M. LAL SPE 54356 Wellbore Stability – Current Performance and Future Challenges,” in Chemicals in the Oil Industry: Developments and Applications, Ed. Ogden, P.H., Royal Soc of Chemistry, London, 1991, pp. 53-70. 15. Horsrud, P, Holt, R. M., Sonstebo, E.: “Time Dependent Borehole Stability: Laboratory Studies and Numerical Simulation of Different Mechanisms in Shale,” paper SPE 28060 presented at the 1994 SPE/ISRM Rock Mechanics in Petroleum Engineering Conference, Delft, 29-31 16. Gazaniol, D., Forsans, T., Boisson, M. J. F. and Plau, J. M.: “Wellbore Failure Mechanisms in Shales: Prediction and Prevention,” JPT, July 1995, pp. 5890595 SI Metric Conversion Factors ft x 3.048* E-01 = m in. x 2.54* E+00 = cm lbm x 4.535 924 E-01 = kg psi x 6.894 757 E+00 = kPa * Conversion factor Fig. 1-Pressure diffusion from wellbore wall with time. Fig. 2-Determining cohesive strength S0, and internal friction angle φ from laboratory strength tests. Fig. 3-Predicted vs measured rock strengths using lab and sonic log velocities with velocity-strength correlation.