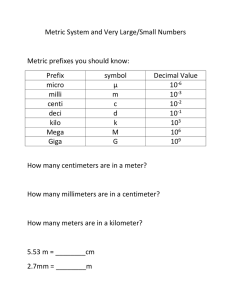
Chemistry 30S Math Skills Name: 1 Exponents An exponent is a shorthand notation for the number of times a number is multiplied by itself. Examples: n4 = n x n x n x n a6 = a x a x a x a x a x a 24 = 2 x 2 x 2 x 2 = 16 32 = 3 x 3 = 9 Negative exponents are shorthand for the inverse of the corresponding positive exponents. Examples: n-4 = 1/n4 = 1/(n x n x n x n) 2 -4 = ½ x ½ x ½ x ½ = 1/16 3-2 = 1/3 x 1/3 = 1/9 Powers of Zero and One Any nonzero number raised by the exponent 0 is 1. Examples: n0 = 1 20 =1 9990=1 Any number raised by the exponent 1 is the number itself. Examples: n1 = n 21 = 2 9991 = 999 Powers of Ten In science, numbers are often written in powers of ten. For numbers greater or equal to one, the exponent on the base 10 equals the number of spaces to the right of the “1” that you place the decimal point. Examples: 100 = 1 101 = 10 102 = 10 x 10 = 100 103= 10 x 10 x 10 = 1 000 2 Negative Powers of Ten Negative exponents equal the inverse of the corresponding positive exponents. For numbers less than one, a negative exponent on the base 10 equals the number of spaces to the left of the “1” that you place the decimal point 10-1 = 1/10 = 0.1 “one tenth” -2 10 = 1/100 = 0.01 “one hundredth” 10-3= 1/1000 = 0.001 “one thousandth” Practice: Evaluate the following 1) 28 = 2) 2-8 = 3) 34 = 4) 105 = 5) 10 -8 = 6) 100= 7) 51 = 8) 60 = 9) 10 -5 = 10) 10-1= 3 Scientific Notation Do you know this number 300,000,000 m/sec? It's the speed of light! Do you recognize this number 0.000 000 000 753 kg? This is the mass of a dust particle! Scientists have developed a shorter method to express very large and very small numbers. This method is called scientific notation. Scientific Notation is based on powers of 10. Distance from Earth to the Sun: 149 600 000 km In scientific notation this number is written as 1.496 x 108 km Charge of one proton: 0.000 000 000 000 000 000 1602 Coulombs In scientific notation this number is written as 1.602 x 10-19 Coulombs A positive exponent indicates that the decimal point was shifted that number of places to the left. A negative exponent indicates that the decimal point was shifted that number of places to the right. Writing numbers in Scientific Notation Scientific Notation is a method of writing numbers in terms of decimal numbers between one and 10 multiplied by a power of ten. There is only one digit to the left of the decimal place, a digit of 1-9. Placeholder zeros are dropped. 0.0005 all zeros are placeholders therefore it is written 5 x 10-4 35,000 all zeros are placeholders therefore it is written 3.5 x 104 1054 this zero is NOT a placeholder therefore it is written 1.054 x 103 Zeros between non zero digits (1-9) are not placeholder zeros. Zeros at the beginning of a number are placeholder zeros. Zeros at the end of a number are placeholder zeros only if they do not follow a decimal. 4 The number 123,000,000,000 in scientific notation is written as: The first number 1.23 is called the coefficient. It must be greater than or equal to 1 and less than 10. The second number is called the base. It must always be 10 in scientific notation. The base number 10 is always written in exponent form. In the number 1.23 x 1011 the number 11 is referred to as the exponent or power of ten. To write a number in scientific notation: 1. Put the decimal after the first digit and drop the non-significant zeroes (placeholder zeros). In the number 123,000,000,000 the coefficient will be 1.23 2. To find the exponent count the number of places the decimal was moved. In 123,000,000,000 the decimal is moved from the end of the last zero on the right 11 places to the left and stops between the 1 and the 2. Therefore we write 123,000,000,000 as: 1.23x1011 Exponents are often expressed using other notations. The number 123,000,000,000 can be found written on your calculator display as: 1.23E+11 or 1.23 X 10^11 For small numbers we use a similar approach. Numbers less than 1 will have a negative exponent. A millionth of a second is: 0.000001 sec. or 1.0E-6 or Examples: Write the following in scientific notation: 1.25 =1.25x100 35 000 = 3.5 x 104 25.78 = 2.578x101 0.0035 = 3.5 x 10-3 100 410 = 1.0041x105 35 000 000 =3.5 x 107 35 = 3.5 x 101 0.35 =3.5 x 10-1 5 Examples 1: Write the following numbers in scientific notation: a) 2 232 000 = b) -0.000 146 = Examples 2: Write the following in standard notation: a) 1.142 × 105 = b) 1.485 × 10-6 = Practice 1: Write the following numbers in scientific notation: a) 1001 = b) 53 = c) 6 926 300 000 = d) -392 = e) 0.003 61 = f) 0.135 92 = g) -0.003 8 = h) 0.000 000 13 = i) -0.567 = j) 0.0137 = Practice 2: Write the following scientific notations in standard notation: a) 1.92 × 103 = b) 3.051 × 101 = c) -4.29 × 102 = d) 6.251 × 109 = e) 8.317 × 106 = f) 1.03 × 10-2 = g) 8.862 × 10-1 = h) 9.512 × 10-8 = i) -6.5 × 10-3 = j) 3.159 × 102 = 6 Using Scientific Notation in Arithmetic Operations Calculate the following: a) 3.12 × 106 * 4.50 × 10-4 ÷ 8.13 × 10-2 = b) (4.1357 × 10-15) (5.4 × 102) = c) d) 4.1357 × 10−15 5.4 × 102 = (3.5 𝑥 102 )(1.5 𝑥102 ) (2.0 𝑥 103 )(5.0 𝑥 101 ) = Practice: Calculate the following: a. 1.695 × 104 ÷ 1.395 × 1015 = b. (4.367 × 105) (1.96 × 1011) = c. 6.97 × 103 * 2.34 × 10-6 + 3.2 × 10-2 = d. 5.16 × 10-4 ÷ 8.65 × 10-8 + 9.68 × 104 = e. f. 3.0 𝑥 105 6.0 𝑥 102 = (3.0 𝑥 102 )(2.0 𝑥102 ) (2.0 𝑥 102 )(4.0 𝑥 104 ) 7 SI Measurement The International System of Units (abbreviated SI from French: Système international d'unités) is the world's most widely used system of measurement and is the measurement system used in science. The SI units we will use in Chemistry 30S include: Second (s) for measuring time Metre (m) for measuring length The mole (mol) for the amount of Metric Prefixes a substance Text Symbol Factor Prefixes used in the SI system are tera T 1 000 000 000 000 shown in the table. giga G 1 000 000 000 Using the meaning of the prefixes, we mega M 1 000 000 can see that: kilo k 1 000 hecto h 100 1 kilometre = 1000 metres deca da 10 1 kilogram = 1000 grams (none) (none) 1 1 centimetre = 0.01 metres 1 deci d 0.1 or of a metre 100 centi c 0.01 1 milligram = 0.001 grams milli m 0.001 1 micro μ 0.000 001 or of a gram 1000 nano n 0.000 000 001 1 Liter = 1000 milliliters pico p 0.000 000 000 001 8 Metric Prefixes _____________________________________________________________________ Prefix Symbol Meaning Number Power of Ten ______________________________________________________________________ Prefixes that increase the size of the unit megaM one million 1 000 000 106 kilo- 1 000 103 Prefixes that decrease the size of the unit decid one tenth 0.1 10-1 centi- c one hundredth 0.01 10-2 milli m one thousandth 0.001 10-3 micro- one millionth 0.000 001 10-6 nano- n one billionth 0.000 000 001 10-9 k one thousand ___________________________________________________________________________________________________________ What are the equivalent measurements for the following? 1. 1 km = ____________m 2. 1 m = _____________cm 3. 1 m= _____________nm 4. 1 cm = ____________mm 5. 1 L = ______________mL 6. 1kg = ______________g 7. 1 hour = _________ min 8. 1 min = _________ s 9. 1 day =__________ hour 10. 1 year = _________ days 9 Conversions of Units Conversion factors are a ratio of equivalent measurements. For example since 1 m = 100 cm we can write the ratio in two ways: 100 𝑐𝑚 1𝑚 and 1𝑚 100 𝑐𝑚 Notice that in a conversion factor the measurement in the numerator (on the top) is equivalent to the measurement in the denominator (on the bottom). Conversion factors are useful in solving problems in which a given measurement must be expressed in some other unit of measure. When a measurement is multiplied by a conversion factor, the numerical value is generally changed, but the actual size of the quantity measured remains the same. Some commonly used conversion factors include: 1𝑚 60 𝑠𝑒𝑐𝑜𝑛𝑑𝑠 1 𝑘𝑔 60 𝑚𝑖𝑛𝑢𝑡𝑒𝑠 100 𝑐𝑚 1 𝑚𝑖𝑛𝑢𝑡𝑒 1000 𝑔 1 ℎ𝑜𝑢𝑟 Practice: Write conversion factors for the following measurements. a) mg and g b) m and km c) day and hours d) L and mL e) Kg and g f) µg and ng 10 Converting Between Units In Chemistry you often need to express a measurement in a unit different from the one given or measured initially. Problems in which a measurement with one unit is converted to an equivalent measurement with another unit are easily solved using conversion factors. Example 1: Convert 12.5 m to its equivalent in cm. Example 2: Write 455 250 mm as its equivalent in kilometers. Example 3: Determine how many minutes there are in 2 days. Example 4: Convert 80 m/s to km/hr 11 Practice: Conversion Factor Calculations 1. Convert 14 mm to its equivalent in m. 2. How many kg is 3 500 grams? 3. Write 57 mg as its equivalent in kilograms. 4. Convert a speed of 20 m/s to its equivalent in km/h. 5. Express 50 km/h in m/s. 12 Directions: Perform the following conversions as indicated. Length 1. 70 cm to m = 2. 49 cm to mm = 3. 8 m to mm = 4. 14.76 m to cm = 5. 8500 cm to m = 6. 250 mm to m = 7. 68.9 cm to mm = 8. 3.25 cm to mm = 9. 59.8 mm to cm = 10. 3.542 mm to cm = 11. 5.3 km to m = 12. 9.24 km to m = 13. 27.500 m to km = 14. 14.592 m to km = 15. 2.4 km to cm = 16. 1.95 km to cm = Mass 17. 6 kg to mg = 18. 4.1 g to ng = 19. 8.7 g to kg = 20. 12.5 g to µg = 21. 925 mg to g = 22. 412 mg to g = 23. 8974 mg to g = 24. 5639 mg to cg = 25. 8.4 g to mg = 26. 2.79 g to mg = 27. 8.64 kg to g = 28. 4.53 mg to g = Volume 17. 6 L to ml = 18. 4.1 L to ml = 19. 8.7 L to ml = 20. 12.5 L to ml = 21. 925 ml to L = 22. 412 ml to L = 23. 8974 ml to L = 24. 5639 ml to L = 25. 8.4 L to ml = 26. 2.79 L to ml = 27. 8.64 ml to L = 28. 4.53 ml to L = 13 Solving Literal Equations Rearrange the equation to solve each formula for the indicated variable. 1. 𝒚 = 𝒙 + 𝒃 (𝑓𝑜𝑟 𝑥) 2. 𝑷 = 𝒂 + 𝒃 – 𝒄 (𝑓𝑜𝑟 𝑏) 3. 𝒚 = 𝟓𝒙 – 𝒃 (𝑓𝑜𝑟 𝑏) 4. 𝑷𝑽 = 𝒏𝑹𝑻 (𝑓𝑜𝑟 𝑇) 5. 𝑷𝑽 = 𝒏𝑹𝑻 (𝑓𝑜𝑟 𝑅) 6. 𝑷𝑽 = 𝒏𝑹𝑻 (𝑓𝑜𝑟 𝑃) 7. 𝑨 = 8. 𝒂 = 9. 𝑺 = 𝒃 ( 𝑓𝑜𝑟 𝑐) 𝒄 (𝒃 – 𝒄) (𝑓𝑜𝑟 𝑏) 𝟐 𝒑−𝒌 (𝑓𝑜𝑟 𝑝) 𝒎 10. °𝑪 = 𝑲 − 𝟐𝟕𝟑 𝟓 11. °C = (°F -32)( ) 𝟗 (𝑓𝑜𝑟 𝐾) (for °F) 14 Rules for Determining Significant Figures in Measurements 1. All nonzero numbers are significant. 2. Zeros may or may not be significant depending on their position in the number. Examples are shown on the table below. Rules Examples # of sig figs. 1. A number is a significant figure if it is a) A nonzero digit 6.5 g 132.34 m b) A zero between significant figures 305 m 2.056 kg 20.0 c) A zero at the end of a decimal number 50. L 28.0 cm 18.00 g 2 5 3 4 3 2 3 4 d) Any digit in a number written in scientific notation 4.0 x 105 m 2 -3 6.70 x 10 g 3 2. A number is not significant if it is a) A zero at the beginning of a decimal number (between a decimal point and a nonzero digit) 0.0008 kg 1 0.0953 m 3 b) A zero used as a placeholder in a large number without a decimal point 750 000 km 2 1 430 000 mm 3 15 Practice: Determine the number of significant figures in the numbers below. Number a) 500 b) 5.00 c) 0.500 d) 5.0 x 102 e) 5 # of sig. figs. Number f) 500.00 g) 0.0005 h) 5 0005 i) 55 000 j) 0.5 # of sig. figs Rules for Rounding Off When doing calculations from measured numbers using a calculator, you get answers that give several digits. However, your answer cannot be more precise than your actual measurements. For example if you are calculating the area of a piece of cloth that measures 6.3 m by 3.4 m, the answer is 21.42 m2. Your measurements only have 2 significant figures; so all four digits cannot be significant. You must round off your final answer to two significant figures giving 21 m2. There are two rules to remember when rounding off numbers: 1. If the first digit to be dropped is 4 or less, it and the following digits are just dropped. Ex. Rounding off 5.3132 to 3 significant figures = 5.31 2. If the first digit to be dropped is 5 or greater, the last retained digit is increased by 1. Ex. Rounding off 15.684 to 3 significant figures = 15.7 16 Practice 1: Round to 1 significant figures. Number Rounded number a) 444.4 b) 6.666 c) 83.241 Number Rounded number d) 65 281 e) 0.12345 f) 0.0002361 Practice 2: Round to 2 significant figures. Number Rounded number a) 444.4 b) 6.666 c) 83.241 Number Rounded number d) 65 281 e) 0.12345 f) 0.0002361 Practice 3: Round to 3 significant figures. Number Rounded number a) 444.4 b) 6.666 c) 83.241 Number Rounded number d) 65 281 e) 0.12345 f) 0.0002361 Practice 4: Round to 4 significant figures. Number a) 444.44 b) 6.6666 c) 83.241 Rounded number Number Rounded number d) 65 281 e) 0.12345 f) 0.00023610 17 Rules for Rounding using Significant Figures When multiplying and dividing, round the answer to the lowest number of significant figures found in the question. For example: 2.2 x 3.25 = 7.15 rounding to 2 sig. figs gives an answer of 7.2 30.5/ 5 = 6.1 rounding to 1 sig fig. gives an answer of 6 Note: Exact numbers, such as the number of people in a room, have an infinite number of significant figures. Exact numbers are made by counting up how many of something are present, they are not measurements made with instruments. Another example of this are defined numbers, such as 1 foot = 12 inches. There are exactly 12 inches in one foot. Therefore, if a number is exact, it DOES NOT affect the accuracy of a calculation nor the precision of the expression. When adding and subtracting, round the answer to the lowest number of decimal places in the question. For example: 2.2 + 3.25 = 5.45 would round to one decimal place 5.5 36.2 – 0.06 = 36.14 would round to one decimal place 36.1 36.222 – 0.06 = 36.14 would round to two decimal places of 36.1 18 Practice: Round your answer accordingly to the rules. 1) 7846 X 92437 X 235.649 X 3300= 2) 583.00 ÷ 83= 3) (57.6 X 3) ÷ (34 X 3.00 X 87.507)= 4) 78.00 + 45.6 + 0.00467 + 39.45 + 276.999= 5) 567.000 - 12= 6) 8597 - 0.l= 7) (3.50 X 105) X [2.8 ÷ (5.4 - 4.09)]= 8) (6.10 X 107 ) + (3 X 107 )= 9) 787 X 3.0= 10) 2.34 x 33.5 = 11) 3.461728 + 14.91 + 0.980001 + 5.2631 = 12) 23.1 + 4.77 + 125.39 + 3.581 = 13) 22.101 - 0.9307 = 14) 0.04216 - 0.0004134 = 15) 564 321 – 264 321 = 16) (3.4617 x 107) ÷ (5.61 x 10¯4) = 17) [(9.714 x 105) (2.1482 x 10¯9)] ÷ [(4.1212) (3.7792 x 10¯5)] = 18) (4.7620 x 10¯15) ÷ [(3.8529 x 1012) (2.813 x 10¯7) (9.50)] = 19) [(561.0) (34 908) (23.0)] ÷ [(21.888) (75.2) (120.00)] = 20) (2.5 x 104)2 = 19 Appendix A: Solutions to Practice Questions Page 3 Answers: 1) 256 2) 0.00390625 3) 81 4) 100 000 5) 0.000 000 01 6) 1 7) 5 8) 1 9) 0.00001 10) 0.1 Page 6 Answers: 1 a) 1.001 X 103 b) 5.3 x 101 c) 6.9263 x 109 d) -3.92 x 102 e) 3.61 x 10-3 f) 1.3592 x 10-1 g) -3.8 x 10-3 h) 1.3 x 10-7 i) -5.67 x 10-1 j) 1.37 x 10-2 2a) 1 920 b) 30.51 c) -429 d) 6 251 000 000 e) 8 317 000 f) 0.010 3 g) 0.886 2 h) 0.000 000 095 12 i)-0.0065 j) 315.9 Page 7 Answers: a) 1.215 × 10-11 c) 4.83 × 10-2 e) 5.0 x 102 b) 8.56 × 1016 d) 1.03 × 105 f) 7.5 x 10-3 Page 9 Answers: 1. 1000 m 2. 100 cm 3. 1 000 000 000 nm 4. 10 mm 5. 1000 ml 6. 1000 g 7. 60 min 8. 60 s 9. 24 hours 10. 365 days 20 Page 10 Answers: 1000 𝑚𝑔 a) 1𝑔 b) c) d) e) f) 1000 𝑚 1 𝑘𝑚 1 𝑑𝑎𝑦 24 ℎ𝑜𝑢𝑟𝑠 1000 𝑚𝑙 1𝐿 1000 𝑔 1 𝑘𝑔 1 µ𝑔 1000 𝑛𝑔 and and and and and and Page 12 Answers: 1. 0.014 m 2. 3.5 kg 1𝑔 1 000 𝑚𝑔 1 𝑘𝑚 1000 𝑚 24 ℎ𝑜𝑢𝑟𝑠 1 𝑑𝑎𝑦 1𝐿 1 000 𝑚𝑙 1 𝑘𝑔 1 000 𝑔 1000 𝑛𝑔 1 µ𝑔 3. 0. 000057 kg 4. 72 km/h 5. 14 m/s 21 Page 13 Answers Length 1. 70 cm to m = 0.7 m 2. 49 cm to mm = 490 mm 3. 8 m to mm = 8 000 mm 4. 14.76 m to cm = 1 476 cm 5. 8500 cm to m = 8.5 m 6. 250 mm to m = 0.25 m 7. 68.9 cm to mm = 689 mm 8. 3.25 cm to mm = 32.5 mm 9. 59.8 mm to cm = 5.98 cm 10. 3.542 mm to cm = 0.3542 cm 11. 5.3 km to m = 5 300 m 12. 9.24 km to m = 9 240 m 13. 27.500 m to km = 0.027500 km 14. 14.592 m to km = 0.014592 km 15. 2.4 km to cm = 240 000 cm 16. 1.95 km to cm = 195 000 cm Mass 17. 6 kg to mg = 6 000 000 mg 18. 4.1 g to ng = 4 100 000 000 ng 19. 8.7 g to kg = 0.0087 kg 20. 12.5 g to µg = 12 500 000 µg 21. 925 mg to g = 0.925 g 22. 412 mg to g = 0.412 g 23. 8974 mg to g = 8. 974 g 24. 5639 mg to cg = 563.9 cg 25. 8.4 g to mg = 8 400 mg 26. 2.79 g to mg = 2 790 mg 27. 8.64 kg to g = 8 640 g 28. 4.53 mg to g = 0.00453 Volume 17. 6 L to ml = 6 000 ml 18. 4.1 L to ml = 4 100 ml 19. 8.7 L to ml = 8 700 ml 20. 12.5 L to ml = 12 500 ml 21. 925 ml to L = 0.925 L 22. 412 ml to L = 0.412 L 23. 8974 ml to L = 8.974 L 24. 5639 ml to L = 5.639 L 25. 8.4 L to ml = 8 400 ml 26. 2.79 L to ml = 2790 ml 27. 8.64 ml to L = 0.00864 L 28. 4.53 ml to L = 0.00453 L 22 Page 14 Answers: 1. 𝑥 = 𝑦 – 𝑏 2. 𝑏 = 𝑃 – 𝑎 + 𝑐 𝑃𝑉 4. 𝑇 = 𝑛𝑅 𝑃𝑉 𝑛𝑅𝑇 5. 𝑅 = 6. 𝑃 = 𝑛𝑇 𝑉 8. 𝑏 = 2𝑎 + 𝑐 9. 𝑝 = 𝑆𝑚 + 𝑘 9 11. °F= °C( )+ 32 3. 𝑏 = 5𝑥 – 𝑦 𝑏 7. 𝑐 = 𝐴 10.K = °C + 273 5 Page 16 Answers: Number a) 500 b) 5.00 c) 0.500 d) 5.0 x 102 e) 5 # of sig. figs. 1 3 3 2 1 Number f) 500.00 g) 0.0005 h) 5 0005 i) 55 000 j) 0.5 # of sig. figs 5 1 5 2 1 Page 17 Answers: Practice 1: Round to 1 significant figures. Number Rounded Number number a) 444.4 400 d) 65 281 b) 6.666 7 e) 0.12345 c) 83.241 80 f) 0.0002361 Practice 2: Round to 2 significant figures. Number a) 444.4 b) 6.666 c) 83.241 Rounded number 440 6.7 83 Rounded number 70 000 0.1 0.0002 Number Rounded number d) 65 281 65 000 e) 0.12345 0.12 f) 0.0002361 0.000 24 23 Practice 3: Round to 3 significant figures. Number Rounded Number number a) 444.4 444 d) 65 281 b) 6.666 6.67 e) 0.12345 c) 83.241 83.2 f) 0.0002361 Practice 4: Round to 4 significant figures. Number a) 444.44 b) 6.6666 c) 83.241 Page 19 Answers: 1) 5.6 X 1014 2) 7.0 3) 0.02 4) 440.1 5) 555 6) 8597 7) 750000 8) 9 X 107 Rounded number 444.4 6.667 83.24 Rounded number 65 300 0.123 0.000236 Number Rounded number d) 65 281 65 280 e) 0.12345 0.1235 f) 0.0002361 0.0002361 9) 2400 10) 78.4 11) 24.61 12) 156.8 13) 21.170 14) 0.04175 15) 3.00000 x 105 16) 6.17 x 1010 17) 13.40 18) 4.62 x 10¯22 19) 2.28 x 103 20) 6.3 x 108 24