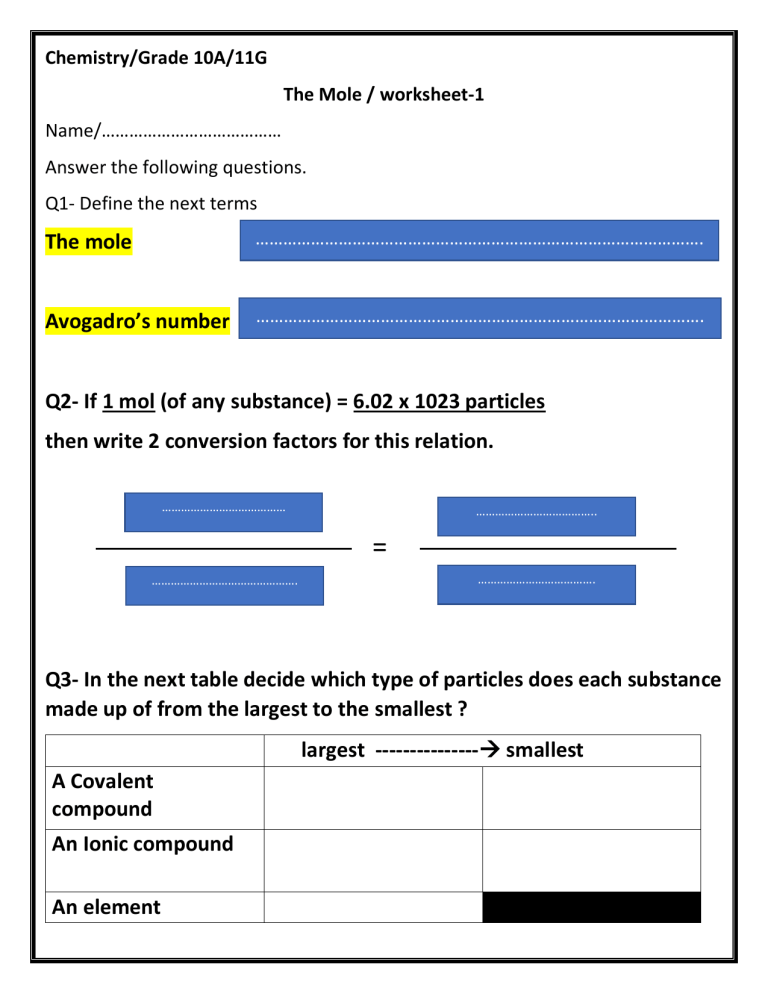
Chemistry/Grade 10A/11G The Mole / worksheet-1 Name/………………………………… Answer the following questions. Q1- Define the next terms The mole ……………………………………………………………………………………. Avogadro’s number ……………………………………………………………………………………. Q2- If 1 mol (of any substance) = 6.02 x 1023 particles then write 2 conversion factors for this relation. ………………………………… ……………………………….. = ………………………………………. ………………………………. Q3- In the next table decide which type of particles does each substance made up of from the largest to the smallest ? largest --------------- smallest A Covalent compound An Ionic compound An element largest --------------- smallest Methane gas CH4 Glucose C6H12O6 Sodium chloride NaCl Iron Fe Q4- Determine the number of atoms in each element from the next list moles 2.5 mol of Zn Calculation 2.5mol Zn ………………. ……………….. 3.2 mol of Cu ………………… ………………. ……………….. 0.25 mol of He ………………… ………………. ……………….. Particles(Atoms) Q4- Determine the number of molecules in each substance from the next list. moles 1.25 mol of CH4 Calculation ………………. Particles(molecules) ………………. ……………….. 3.2 mol of C6H12O6 ………………… ………………. ……………….. Q5- Determine the number of moles in each substance from the next list. particles 1.807x 1024 atom of Ar Calculation ……………… ………………. ……………….. 1.5055x1023 molecules of H2O ………………… ………………. ……………….. moles





