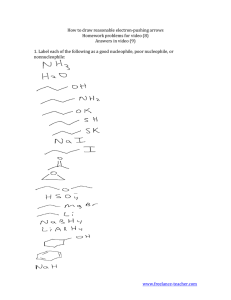
Nucleophilic Reactions Properties and Substitution Reactions of Alkyl halides John Wilfred T. Malabanan RPh Has a halogen atom bonded to an sp3-hybridized carbon atom Carbon-halogen bond in an alkyl halide is polartized Alkyl Halides because the halogen is more electronegative than carbon. Therefore, the carbon atom has a partial positive charge (∂+) and the halogen has a partial negative charge (∂-) Alkyl halides are classified int primary, secondary and tertiary Aryl halides = aromatic ring with halogen substituent Alkenyl Halide = Halogen double bonded to a carbon Phenyl Halide = aromatic ring is a benzene Alkyl halides are poorly Physical Properties of Alkyl Halide soluble in water Chloroalkanes are carcinogenic and should be used under the fumehood. Nucleophile (Nu:) displaces a leaving group (LG) in the molecule that undergoes substitution, Nucleophilic Substituion Nucleophile = always a Lewis Base (electron Pair Donor); may be negatively charged or neutral. Leaving grou = Lewis Acid ( electron Pair Acceptor) Often substrate is an alkyl halide (R-X) and the leaving group is a halide anion (X) The nucleophile uses its electron pair to form a new covalent bond with the substrate carbon. Heterolytic bond cleavage -happens in the bond between the substrate and the leaving group -the unshared electron pair of the nucleophile forms a new bond to the carbon atom. Lewis Base Nu(-) + R-LG ----- R-Nu + LG(-) The bond between the carbon and the leaving group breaks, giving both electrons from the bond to the leaving group. The leaving leaving group gains the pair of electrons that originally bonded it in the substrate. Nucleophilic Substitution by a Negatively Charged Nucleophile Results in a Neutral product Solvolysis -the nucleophile is a solvent molecule. Since solvent molecules are present in great excess the equilibrium favors the transfer of proto from the alkyloxonium ion to a water molecule. OH + R-X ------> H-O-R + X(-) Nucleophilic Substitution by a Neutral Nucleophile Results initially in a positively charged product. H-O-H + R-X ----> H2O(+)R + X(-) Then it will under go proton transfer and yield a netral product of H-O-R + H3O(+) +X(-) To act as a substrate the nucleophilic substituition Leaving group reaction must have a good leaving group A Good Leaving group is a substituent that can leave a relatively stable, weakly basic molecule or ion CH3-Cl+OH ---- CH3OH + Cl(-) Rate of Reaction = (CH3Cl)(OH) The reaction is said to be in the Sn2 Reaction (substitution, nucleophilic, bimolecular) second over all order. OH and CH3Cl must collide. Bimolecular reaction – two species are involved Molecularity - the number of species involved in a reaction step. • Nucleophile approaches the carbon bearing the leaving Mechanism of SN2 Reaction group form the back side directly opposite the leaving group • As the nucleophile froms a bond and the elaving group departs, the substrate carvon atom undergoes inversion • SN2 reaction proceeds in a single step (without intermediates) through an unstable arrangement of atoms called the transition state. Free Energy of activation - difference in energy between rge reactants and the transition stage Free Energy change for the reaction – difference between the reactants and the products Mechanism for SN1 Reaction It is the nucleophilic substitution in which the nucleophile is a molecule of the solvent. Solvolysis Reaction When the solvent is water we call the reaction hydrolysis. When the solvent is methanol we call it mathanolysis. 1. Structure of the substrate Factors affecting the relative rates of SN1 and SN2 reactions. Concentration and reactivity of the nucleophile Effect of solvent Nature of leaving group Steric Effect – an effect of the relative rates caused by the space-filling properties of those parrs of a molecule attached at or near the reacting site. Steric Hindrance – when the spatial arrangement Structure of the substrate of atoms or groups at or near a reacting site of a molecule hinders or retards a reaction. Hamond-Leffler postulate – the transition-state structure for an uphill energy step should show a strong resemblance to the product structure from that step. A negatively charged nucleophile is always a more reactive nucleophile than its conjugate acid. HO (-) > H2O and RO (-) > ROH Concentration and Strength Ina group of nucleophiles in which the nucleophilic atom is the same, nucleophilicities parallel basicities. RO- > HO- >> RCO2- >ROH > H2O When the nucleophilic atoms are different, nucleophilicities may not parallel basicities. HS- > N=C- > I- >HO- Polar aprotic Solvents – SN2 Solvent Effects -solubilize cations well using their unshared electron pairs but do not interact as strongly with anions because they cannot hydrogen bond with them and because the positive regions of the solvent are shielded by steric effects from the anion. - Acetone, DMSO, DMF, HMPA Polar protic Solvents – SN1 -has atleast one hydrogen atom capable of participating in a hydrogen bond Solvent Effects -EtOh, MeOH facilitate the formation of a carbocation by forming hydrogen bonds with the leaving group as it departs, thereby lowering the energy of the transition state leading to a carbocation. General trend of halide nucleophilicity in polar aprotic solvents: F > Cl > Br > I General trend of halide nucleophilicity in polar protic solvents: I > Br > Cl > F Larger atoms has greater polarizabilitym A substrate that can form a relatively stable carbocation A realtively weak nucleophile A polar, protic solvent such as EtOH, MeOH or H2O Summary of SN1 reactions The SN! Mechanism is therefore important in solvolysis reactions of tertiary alkyl halides, especially when the solvent is highly polar. In a solvolysis reaction the nucleophile is weak because it is a neutral molecue rather than an anion. A substrate with a relatively unhindered leaving group Summary of SN2 Reaction Tertiary halides do not react by SN2 mechanism High concentration of the nucleophile A polar, aprotic solvent Synthesis of Alkene: Elimination reactions Most important means for synthesizing alkenes. In an elimination reaction the fragments of some molecule are removed (eliminated) from adjacent atoms of the reactant. Elimination reaction Dehydrohalogenation Homework: Give a List of Strong Bases used in Dehydrohalogenation Widely used method of synthesizing alkenes via the elimination of HX from adjacent atoms. The best reaction conditions to use when synthesizing an alkene by dehydrohalogenation are those that promote an E2 mechanism. Dehydrohalogenation In an E2 mechanism, a base removes a b hydrogen from the b carbon, as the double bond forms and a leaving group departs from the a carbon. Use a secondary or tertiary alkyl halide if possible. Why? Because steric hindrance in the substrate will inhibit substitution. When a synthesis must begin with a primary How To Favor an E2 Mechanism alkyl halide, use a bulky base. Why? Because the steric bulk of the base will inhibit substitution. Use a high concentration of a strong and nonpolarizable base such as an alkoxide. Why? Because a weak and polarizable base would not drive the reaction toward a bimolecular reaction, thereby allowing unimolecular processes (such as SN1 or E1 reactions) to compete. Sodium ethoxide in ethanol (EtONa/EtOH) and How To Favor an E2 Mechanism potassium tert-butoxide in tert- butyl alcohol (tBuOK/t-BuOH) are bases typically used to promote E2 reactions Why? Note that in each case the alkoxide base is dissolved in its corresponding alcohol. (Potassium hydroxide dissolved in ethanol or tertbutyl alcohol is also sometimes used, in which case the active base includes both the alkoxide and hydroxide species present at equilibrium.) Use elevated temperature because heat How To Favor an E2 Mechanism generally favors elimination over substitution. Why? Because elimination reactions are entropically favored over substitution reactions Potassium Hydroxide dissolved in Bases Used in Dehydrohalogenation ethanol (KOH/EtOH) = sometimes used Conjugate bases of Alcohols, such as sodium ethoxide (EtONa) is more advantageous. The conjugate base of an alcohol (an alkoxide) can be prepared by treating an alcohol with an alkali metal. Bases Used in Dehydrohalogenation Alcohol Sodium Alkoxide Oxidation-reduction Sodium reacts with Oxygen atoms to generate Hydrogen gas and the alkoxide anion. Sodium alkoxides can also be prepared by allowing an alcohol to react with NaH. Bases Used in Dehydrohalogenation RO-H + Na:H R-O-Na+ +H2 Bases Used in Dehydrohalogenation Sodium (and potassium) alkoxides are usually prepared using an excess of the alcohol, where the excess alcohol becomes the solvent for the reaction. Sodium ethoxide is frequently prepared this way using ethanol Potassium Tert-butoxide (t-BuOK) is another highly effective base dehydrohalogenation. It can be made by: Bases Used in Dehydrohalogenation Formation of the More Substituted Alkene is Favored with a Small Base. Zaitsev’s Rule Dehydrohalogenation of many alkyl halides, however, yields more than one product. For example, dehydrohalogenation of 2-bromo-2methylbutane can yield two products: 2-methyl-2-butene and 2-methyl-1-butene, as shown here by pathways (a) and (b), respectively: Zaitsev’s Rule If we use a small base such as ethoxide or hydroxide, the major product of the reaction will be the more highly substituted alkene (which is also the more stable alkene). Zaitsev’s Rule The reason for this behavior is related to the double-bond character that develops in the transition state for each reaction Zaitsev’s Rule Formation of the Less Substituted Alkene Using a Bulky Base Zaitsev’s Rule When an elimination yields the less substituted alkene, we say that it follows the Hofmann rule. The five atoms involved in the transition state of an E2 reaction (including the base) must be coplanar, i.e., lie in the same plane. Stereo Chemistry of E2 Reactions The anti coplanar conformation is the preferred transition state geometry Syn coplanar transition state occurs only with rigid molecules that are unable to assume the anti arrangement. Mechanism of E2 Reaction The E1 Reaction The E1 Reaction If a solvent molecule reacts as a nucleophile at the positive carbon atom of the tert-butyl cation, the product is tert-butyl alcohol or tertbutyl ethyl ether and the reaction is S 1 N If, however, a solvent molecule acts as a base and removes one of the b hydrogen atoms, the product is 2-methylpropene and the reaction is E1 Most alcohols undergo dehydration (lose a molecule of water) to form an alkene when heated with a strong acid. Dehydration of Alcohols 1. The temperature and concentration of acid required to dehydrate an alcohol depend on the structure of the alcohol substrate. (a) Primary alcohols are the most difficult to dehydrate. Dehydration of ethanol, for example, requires concentrated sulfuric acid and a temperature of 180 8C: 1. The temperature and concentration of acid required to dehydrate an alcohol depend on the structure of the alcohol substrate. (a) Primary alcohols are the most difficult to Dehydration of Alcohols dehydrate. Dehydration of ethanol, for example, requires concentrated sulfuric acid and a temperature of 180 C: 1. The temperature and concentration of acid required to dehydrate an alcohol depend on the structure of the alcohol substrate. Secondary alcohols usually dehydrate under milder conditions. Cyclohexanol, for example, dehydrates in 85% phosphoric acid at 165–170 C: Dehydration of Alcohols 1. The temperature and concentration of acid required to dehydrate an alcohol depend on the structure of the alcohol substrate. Dehydration of Alcohols Tertiary alcohols are usually so easily dehydrated that relatively mild conditions can be used. tert-Butyl alcohol, for example, dehydrates in 20% aqueous sulfuric acid at a temperature of 85 C: Dehydration of Alcohols 2. Some primary and secondary alcohols also undergo rearrangements of their carbon skeletons during dehydration. Such a rearrangement occurs in the dehydration of 3,3-dimethyl-2-butanol: Dehydration of Alcohols 2. Some primary and secondary alcohols also undergo rearrangements of their carbon skeletons during dehydration. Such a rearrangement occurs in the dehydration of 3,3-dimethyl-2-butanol: Dehydration of Alcohols Dehydration of Alcohols Addition of Halogens to alkenes Addition of Hypophalous Acids to Alkenes: Halohydrin Formation Addition of Hypophalous Acids to Alkenes: Halohydrin Formation Addition of Water to Alkenes: Oxymerucration Addition of Water to Alkenes: Oxymerucration Addition of Water to Alkenes: Hydroboration Addition of Water to Alkenes: Hydroboration Addition of Carbenes: Cyclopropene Synthesis Addition of Carbenes: Cyclopropene Synthesis Addition of Carbenes: Cyclopropene Synthesis Addition of Carbenes: Cyclopropene Synthesis Simmons-Smith Reaction: Addition of Carbenes: Cyclopropene Synthesis Reduction of Alkenes: Hydrogenation Platinum is usually used as PtO2 or Adam’s Catalyst Reduction of Alkenes: Hydrogenation Platinum is usually used as PtO2 or Adam’s Catalyst Reduction of Alkenes: Hydrogenation Reduction of Alkenes: Hydrogenation Oxidation of Alkenes :Epoxidation and Hydroxylation Epoxide = Oxirane -a cyclic ether with an oxygen atom in a three membered ring. Oxidation of Alkenes :Epoxidation and Hydroxylation Oxidation of Alkenes :Epoxidation and Hydroxylation Oxidation of Alkenes: Cleavage to Carbonyl Compounds