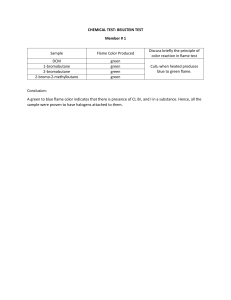
Atomic Absorption Spectrometer Varian SpectrAA Model 220FS The Instrument The instrument consists of: 1. A flame 2. Lamps to produce the correct wavelength of light 3. A detector 4. A system to aspirate solutions into the flame 5. A computer to control the experiment The Instrument On the left is the flame (behind the grid) and the spectrometer. The two bottles contain water used for flushing the tubing and for diluting solutions that are too concentrated. The round object is a pump. On the right is a cabinet containing the lamps shown on a later slide. The flame, like all large burners, is vented at the top. The Lamps From bottom to top, the lamps are for Mg, Ca, K, and a combination of Fe, Co, Ni, Mn, Cu, and Cr. Each element uses a specific wavelength of light. The Flame The flame is with only water being aspirated. The two holes, left and right, are where the light beam enters and leaves after passing through the flame. The dark place at the top is a stain from the heat of the flame. The Instrument Current spectrometers use a PC Computer to control the experment. There needs to be standards (solutions of known concentration) to calibrate the instrument. The experiment must be setup in the program controlling the experiment with • Ions to be analyzed • Concentration of the standards • Number of points to be measured • Wavelength of light • Lamp Position Measurement - Standards A set of standards ready to be aspirated into the flame. This instrument automatically dilutes the solution. Aspiration of the Solution Being Measured A sample of maple syrup ready to be aspirated into the flame. The PC Screen The solution being measured has an absorbance of 0.068 which corresponds to a concentration of 10.2 ppm Colors Produced by Different Ions The following slides show the colors of different ions in the flame. The differences in intensity of the colors is, in part, due to differences in concentration. The Calcium Flame The calcium flame is red. This is intensely red because the calcium content is high. The Copper Flame The Potassium Flame The Manganese Flame The Cobalt Flame Results The computer stores the data which can be printed. The experiment can be set up to show the calibration curve and the concentrations on the screen. To get reliable concentrations, the program must be told what fitting algorithm to use. As can be seen on the screen shown previously, the calibration data are not linear in that instance.