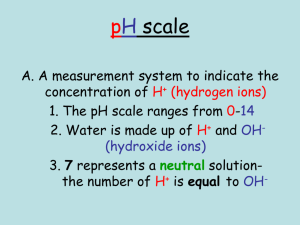
Complete the following questions using the pHET simulation embedded in the canvas page or on the link included. Instructions in how to access the parts of the simulation you need to answer the questions have been provided. You can write responses in your book or print this sheet out and answering the questions. pH Investigation Instructions: To obtain the data for table 1 below you will need to: 1. Click on the “Macro” box. 2. Investigate the pH of each of the following substances. a) Drag the pH sensor into the solution to see the pH reading. b) Record the pH of the substance and whether it is an acid or a base according to the pH scale. c) To change the substance simply select from the drop-down menu. Instructions: 1. Complete the table using the simulation. Substance Drain cleaner Hand soap Blood Spit Milk Chicken Soup Coffee Orange Juice Soda Pop Vomit Battery Acid pH acid/base 1. Using the information from the chart and simulation answer the following questions. a) What pH values correspond to acids? b) What pH values correspond to bases? 2. Can you predict what a solution with a pH of 7 will look like at the particulate level? Draw a particle diagram of a neutral solution in the box. 3. Would you classify this solution as an acid or base? Explain why, including your reasoning. 4. What effect does adding water have on the pH of acid and base solutions? 5. What are the outer limits of the pH scale? How low and high does it go and what does this mean? My Solution Investigation Instructions 1. In the pHET simulation from activity 1, click the “My Solution” box. 2. Click on the H3O+/OH- ratio box located below the beaker with the substance. 4. Determine the range of the pH scale can cover by adjusting the pH value. Questions: 1. What is the lowest pH value you could achieve in this simulation? 2. Draw a particle diagram of this solution. Is this solution very acidic or very basic? 3. What is the highest pH value you could achieve in the simulation? 4. Draw a particle diagram of this solution. Is this solution very acidic or very basic? 5. Adjust the pH scale so that you have a solution with a pH of 7.00. a. Draw a particulate level diagram of this solution. b. What are the concentrations of H3O+ and OH- ions at? c. Is this solution acidic or basic? Why? Micro Investigation Instructions 1. In the pHET simulation from activity 1, Navigate to the “Micro” box. 2. Click on the H3O+/OH- ratio box located below the beaker with the substance. 3. Investigate how adding more of the substance with the red button on the dropper changes the pH of the resulting solution. Questions: 1. Fill in the data table with data from 5 different solutions (make sure you include at least two acids and two bases). Substance Volume (Liters) Initial pH Final Initial Final 2. What happens to the pH of the solution as you add more of the substance to it? 3. How does adding water to an acid change its’ pH? 4. What happens to the concentration of both H3O+ and OH- ions as water is added to an acid? 5. What happens to the pH of a base as water is added? 6. What happens to the concentration of both H3O+ and OH- ions as water is added to a base? 7. As the pH approaches 0, what happens to the concentration of H3O+ ions? 8. As the pH approaches 0, what happens to the concentration of OH- ions? 9. As the pH approaches 14, what happens to the concentration of H3O+ ions? 10. As the pH approaches 14, what happens to the concentration of OH- ions?