Plastics: Types, Properties & Uses - Chemistry Presentation
advertisement

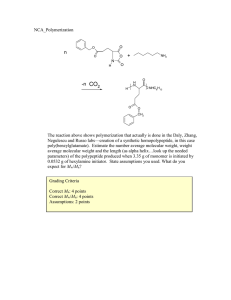
PLASTIC CH 203 Chemical Process Industries INTRODUCTION TO PLASTIC: PLASTIC IS DEFINED AS ORGANIC POLYMER OF HIGH MOLECULAR MASS. THEY ARE USUALLY SYNTHETIC MOST COMMONLY DERIVED FROM PETROCHEMICALS. PLASTICS ARE USUALLY CLASSIFIED INTO THERMOSETTING AND THERMOPLASTIC. THERMOSETTING : THERMOSEETING PLASTICS ARE THOSE PLASTICS THAT CANNOT BE REMOULDED.SUCH AS BAKELITE,POLYAMIDE. THERMOPLASTIC: THERMOPLASTICS ARE THOSE WHICH CAN BE REMOULDED BY HEATING THEM SUCH AS POLYETHYLENE,POLYSTYRENE. 1.POLYETHYLENE: IT IS THE MOST COMMON PLASTIC WITH PRODUCTION OF 80 MILLION METRIC TONS PER YEAR.IT IS MANUFACTURED BY THE POLYMERIZATION OF ETHENE MONOMER UNDER HIGH OR LOW PRESSURE IN THE PRESENCE OF ORGANIC PEROXIDE AS CATALYST WITH TEMPERATURE RANGING FROM 100°-300°C. PROPERTIES AND USES: IT IS A THERMOPLASTIC HAVING MELTING POINT RANGING FROM 105° TO 130°C . THEY HAVE EXCELLENT CHEMICAL RESISTANCE. BURNS SLOWLY WITH A BLUE FLAME . PE IS SOLUBLE IN AROMATIC HYDROCARBONS . IT IS USED IN MAKING POLYETHENE BAGS , BOTTLES. 2.POLYPROPYLENE: POLYPROPYLENE IS FORMED BY THE POLYMERIZATION OF PROPYLENE MONOMER. IT IS CURRENTLY BEING MANUFACTURED BY THREE PROCESSES NAMELY HYDROCARBON SLURRY, BULK SLURRY, GAS PHASE.IN 2008 , POLYPROYLENE MARKET HAD A VOLUME OF 45.1 MILLION METRIC TONS. PROPERTIES AND USES: IT IS TOUGH,FLEXIBLE AND RESITANT TO FATIGUE.THERMOPLASTIC WITH A MELTING POINT RANGING FROM 130° TO 171°C.USED IN BOPP,DIELECTRIC IN CAPACITOR,FOOD CONTAINERS,CAR BATTERIES,WASTE BASKETS. 3.POLYSTYRENE: POLYSTYRENE IS FORMED BY THE POLYMERIZATION OF STYRENE MONOMER.IT IS VERY INEXPENSIVE RESIN. IT IS ONE OF THE MOST WIDELY USED PLACTICS WITH THE PRODUCTION OF SEVERAL BILLION KILOGRAMS PER YEAR. PROPERTIES AND USES: IT IS HARD, BRITTLE HAVING MELTING POINT OF 100°C. IT IS TRANSPARENT BUT CAN BE COLOURED BY PIGMENTS.IT IS USED IN MAKING CD,DVD COVERS , DISPOSABLE CUTLERY , SAFE FOR USE IN FOOD CONTAINERS. 4. BAKELITE: IT IS A THERMOSETTING PLASTIC FORMED BY THE ELIMINATION REACTION OF PHENOL WITH FORMALDEHYDE. IT WAS DEVELOPED BY CHEMIST LEO BAEKELAND IN 1907. BAKELITE WAS ONE OF THE FIRST PLASCTICS TO BE MADE FROM SYNTHETIC COMPOUNDS. IT WAS DESIGNATED A “NATIONAL HISTORIC CHEMICAL LANDMARK” IN 1933 BY” AMERICAN CHEMICAL SOCIETY”. STRUCTURE OF BAKELITE PROPERTIES AND USES: IT IS A THERMOSETTING PLASTIC HAVE EXCELLENT INSULATING PROPERTIES, GOOD MECAHANICAL AND IMPACT STRENGTH .IT IS USED IN MAKING ELECTRICAL SWITCHES, BUTTONS, WIRE INSULATION ,BRAKE PADS ,AUTOMOTIVE COMPONENTS. QUESTIONS AND ANSWERS: Q1. WHAT ARE PLASTICS ? CLASSIFY THEM ? A . PLASTIC IS DEFINED AS ORGANIC POLYMER OF HIGH MOLECULAR MASS. THEY ARE USUALLY SYNTHETIC MOST COMMONLY DERIVED FROM PETROCHEMICALS. PLASTICS ARE USUALLY CLASSIFIED INTO THERMOSETTING AND THERMOPLASTIC.THERMOPLASTICS ARE THOSE WHICH SOFTEN ON HEATING AND HARDEN ON COOLING. THERMOSETTING PLASTICS ARE THOSE WHICH ARE HEAT CURING. Q2. GIVE ADVANTAGES, DISADVANTAGES OF PLASTICS? A . ADVANTAGES: PLASTICS ARE CORROSION RESISTANT AND ARE INERT TO CHEMICAL ATTACKS.THEY HAVE GOOD INSULATING PROPERTIES. THEY ARE EASY TO MOLD. THEY CAN BE COLOURED TO DESIRED COLOURS. THEY ARE LIGHT IN WEIGHT. THEY ARE LONG LASTING AS COMPARED TO METALS. DISADVANTAGES: THEY ARE NOT BIO-DEGRADEABLE THUS CREATING POLLUTION HAZARDS.THEY PRODUCE TOXIC FUMES WHEN BURNT IN DEBRIS. SOME OF THEM ARE NOT SUITABLE FOR FOOD STORAGE PURPOSES. Q3. DEFINE TWO POLYMERIZATION REACTIONS USED IN THE PRODUCTION OF PLASTIC? A . TWO TYPES OF POLYMERIZATION REACTIONS ARE: 1 . ADDITION POLYMERIZATON: IN THIS TYPE OF POLYMERIZATION, SINGLE MONOMER POLYMERIZES ITSELF IN THE PRESENCE OF A CATALYST TO GIVE RESPECTIVE POLYMER. THERMOPLASTICS ARE FORMED BY THIS TYPE OF REACTION. 2 . CONDENSATION POLYMERIZATION: IN THIS TYPE OF POLYMERIZATION TWO DIFFERENT MONOMERS OR SMALLER MOLECULES COMBINE TO GIVE A LARGE MOLECULE OR POLYMER WITH THE ELIMINATION OF SMALLER MOLECULE. THERMOSETTING PLASTICS ARE FORMED BY THIS TYPE OF POLYMERIZATION.