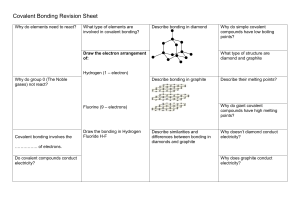
9th GRADE CHEMISTRY WORKSHEET Covalent Compounds 1. a) What is the difference between lone pair and bonding pair electrons? b) Explain what is meant by covalent bonding. c) How is covalent bonding different to Ionic bonding? 2. Circle the pairs of atoms that will bond covalently. (You may refer to the periodic table) a) Mg with Cl b) Li with S c) F with P d) O with N e) F with Cl f) Ni with Rn 3. Draw a Lewis dot structure diagram for each of the following substances;( 1H , 5B, 6C,15P, 17Cl) a) Chlorine Cl2. b) Boron trihydride BH3. c) The compound formed between Carbon(C) and hydrogen(H). d) The compound formed between Phosphorus (P) and hydrogen(H). 4. a) Draw Lewis dot structure diagrams to illustrate the bonding in; (i) magnesium chloride (MgCl2) (ii) carbon tetrachloride(CCl4) 5. Sodium oxide (Na2O), carbon dioxide (CO2) and silicon dioxide (SiO2) are all poor conductors of electricity when solid, but only sodium oxide becomes an excellent conductor when molten. a) Explain why these three compounds don’t conduct electricity when solid. b) Explain why only sodium oxide conducts electricity when molten.