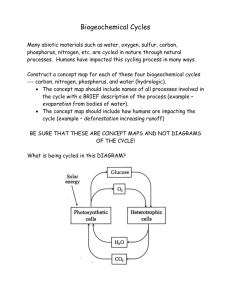
Biogeochemical Cycles: The Flow of Matter LS2.2 •Create a model tracking carbon atoms between inorganic and organic molecules in an ecosystem. Explain human impacts on climate based on this model. LS2.3 • Analyze through research the cycling of matter in our biosphere and explain how biogeochemical cycles are critical for ecosystem function. Biotic and Abiotic Factors •Biotic factors: Living factors of the environment • Examples: animals, plants, fungi, bacteria, protists •Abiotic factors: the nonliving parts of an environment. • Examples temperature, moisture, light, and soil. 2 ENERGY & MATTER Energy flows through the ecosystem in one direction and then gets used up. But…other materials move through the ecosystem too. Atoms are never destroyed . . . Only transformed. Take a deep breath. The atoms you just inhaled may have been inhaled by a dinosaur millions of years ago. Four different elements (atoms) make up 95% of the body in most organisms The same molecules are passed around again and again within the biosphere (Earth’s atmosphere) in Biogeochemical Cycles. Biogeochemical Cycles – Materials are recycled between the biotic and abiotic environment. – The same materials can be reused. Examples of Cycles: • • • • Carbon Nitrogen Phosphorus Water Carbon Cycle Why is carbon important? Found in all the building blocks of cells: • carbohydrates, proteins, nucleic acids, lipids CHO CHON CHONP CHO Carbon Cycle Why is carbon important? Carbon in CO2 provides the atoms for glucose production during photosynthesis… • glucose is the fuel that all living things depend on. Carbon Cycle Why is carbon important? ALL living things break down carbohydrates during cellular respiration (releasing CO2 & water) Decomposers release CO2 into the atmosphere when they break down organic molecules 4 main CARBON Reservoirs (storage): 1.In the atmosphere as CO2 gas 2.In ocean as dissolved CO2 gas 3.On land in organisms, rocks, soil 4.Underground as coal & petroleum (fossil fuels) and calcium carbonate in rocks CO2 in atmosphere CO2 in Ocean BIOLOGY; Miller and Levine; Prentice Hall; 2006 Where does CO2 in atmosphere come from? 1.Volcanic Activity 2.Human Activity (burning fossil fuels, deforestation etc.) 3.Cellular respiration 4.Decomposition of dead organisms CO2 in atmosphere CO2 in Ocean BIOLOGY; Miller and Levine; Prentice Hall; 2006 Human Impacts on the carbon cycle • Greenhouse Gases – gases in the Earth’s atmosphere that trap heat. – They let sunlight pass through the atmosphere, but they prevent the heat that the sunlight brings from leaving the atmosphere. • The main greenhouse gases are: – Water vapor (H2O) – Carbon dioxide (CO2) Human Impacts on the carbon cycle 1. Fossil Fuel burning – releases CO2 to the atmosphere. – Examples: coal, oil 2. Human-driven changes in land use and land cover: – Deforestation (logging, clearing, burning trees) – Urbanization – shifts in vegetation patterns Human Impacts on the carbon cycle 3. Increased biomass – human population is growing exponentially – more people doing cellular respiration (output is CO2) Human Impacts on the carbon cycle 4. Ice caps melting – releases CO2 5. Oceans warming less phytoplankton (autotroph) less photosynthesis (less CO2 removed from atmosphere, less O2 for other life.) Human Impacts on Climate (and the carbon cycle) Why does this matter and what can we do??? 1. Research how these human activities impact climate change. We know we are affecting the carbon cycle, but what is this doing to earth’s climate? 2. Based on the human activities we talked about, what can humans do to reduce (or even reverse) these affects on climate change? Biogeochemical Cycles: The Flow of Matter LS2.3 • Analyze through research the cycling of matter in our biosphere and explain how biogeochemical cycles are critical for ecosystem function. Biogeochemical Cycles – Remember, materials are recycled between the biotic and abiotic environment. – The same materials can be reused. Examples of Cycles: • • • • Carbon Nitrogen Phosphorus Water WHY IS NITROGEN IMPORTANT? • Nitrogen bases make DNA and RNA • Adenine is used in ATP • Nitrogen makes the “amino” part of Amino Acids (monomer of proteins). 79% of the atmosphere is made up of NITROGEN gas (N2) BUT we CAN’T use the nitrogen gas we breathe! The bond in N2 gas is so strong it can only be broken by: Lightening Specialized bacteria Volcanic Activity Bacteria that live in the soil and in symbiotic relationships with plants, called legumes, take nitrogen from the atmosphere and turn it into ammonia (NH3), a form that is usable by plants. This process is called: Nitrogen Fixation Other bacteria in the soil convert ammonia into nitrates (NO3- ) & nitrites (NO2-) which plants can use, take up through their roots. The nitrogen animals need for proteins, ATP, and nucleic acids comes from the food we eat, not the air we breathe! Bacteria that live in the soil also carry out the reverse process Nitrates & nitrites → nitrogen gas This is called Denitrification Nitrogen Cycle • Nitrogen Cycle: – Organisms must have nitrogen to produce amino acids / proteins (CHON) and DNA (CHONP) – Living things cannot use nitrogen gas in the air – Life is possible due to nitrogen-fixation • Nitrogen Fixation: Nitrogen gas is converted to ammonia or other usable nitrogen source. • Done by bacteria or lightening, and sometimes volcanic activity Nitrogen Cycle WHY IS PHOSPHORUS IMPORTANT? Makes DNA and RNA Transfers energy as ATP Makes phospholipids for cell membranes Phosphorous Cycle The only biogeochemical cycle that DOES NOT involve the atmosphere. Biotic • Plants – pulled up through roots • Animals – from eating other living things Abiotic • Most Phosphorous is found in rocks and sediment • Dissolved in freshwater • Ocean sediments Released by: • Death and decay Released by: • Weathering/erosion Water Cycle • Cycle of processes by which water circulates between the oceans, atmosphere, and land • Involves precipitation as rain and snow • Drainage into streams and rivers • Return to the atmosphere by evaporation and transpiration (loss of water from plant leaves). Water Cycle