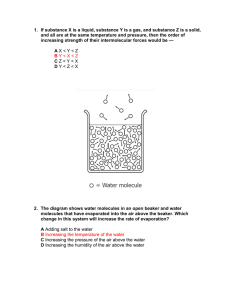
Name ___________________________ Chemistry and the Environment Is Recycling Energy-Efficient? A soda can is made out of 17.73 g of aluminum metal. Most communities have programs in place to recycle aluminum, and a soda can may be back on a shelf ~60 days after collection. 1. Recycling Aluminum To purify an aluminum can, it must be melted. After melting, the can is formed from molds into the final shape. a. If aluminum (C = 0.902 J/g·°C) has a melting point of 655°C, how much energy (in kJ) does it take to heat the can to the melting point from room temperature of 25°C? b. Once at the melting point, the aluminum can absorbs heat, changing state without changing temperature. If the energy needed to melt the can is 10.7 kJ per one mole of aluminum, how much energy does it take to melt the heated can? c. How much total energy does it take to heat the can and then melt the aluminum can for purification? 2. Extracting Aluminum New aluminum can be produced by decomposing Al2O3 in the elements that make it up. Write the balanced chemical reaction for this process. How much energy is required to extract the 17.73 grams needed for the aluminum can if the above reaction requires 3350 kJ for the number of moles of aluminum in the balanced reaction? 3. Comparison Is recycling aluminum energy-efficient? Justify your answer Alternative Fuels In recent times, some engines have been built that use alternative fuel sources to provide the energy the motor needs. The hope is that these new designs will limit the release of carbon dioxide, the greenhouse gas most responsible for climate change. 1. Gasoline Historically, cars have used gasoline, a petroleum mixture, to power cars. Octane (C8H18) is most common substance in gasoline. a. What is the balanced equation for the combustion of octane? b. If the reaction above releases 5075 kJ of energy per the number of moles in the balanced reaction what mass of carbon dioxide is released for every 1000 kJ of energy produced by the engine? 2. Ethanol The Midwest ferments corn to produce ethanol (C2H5OH) as fuel for cars. a. What is the balanced equation for the combustion of ethanol? c. If the reaction above releases 1330 kJ of energy per the number of moles in the balanced reaction what mass of carbon dioxide is released for every 1000 kJ of energy produced by the engine? 3. Natural Gas The United States Postal Service fleet includes hundreds of vehicles that run on natural gas, mostly composed of methane (CH4). a. What is the balanced equation for the combustion of methane? d. If the reaction above releases 802 kJ of energy per the number of moles in the balanced reaction what mass of carbon dioxide is released for every 1000 kJ of energy produced by the engine? 4. Comparison Which fuel has the least impact upon the environment? Justify your answer.