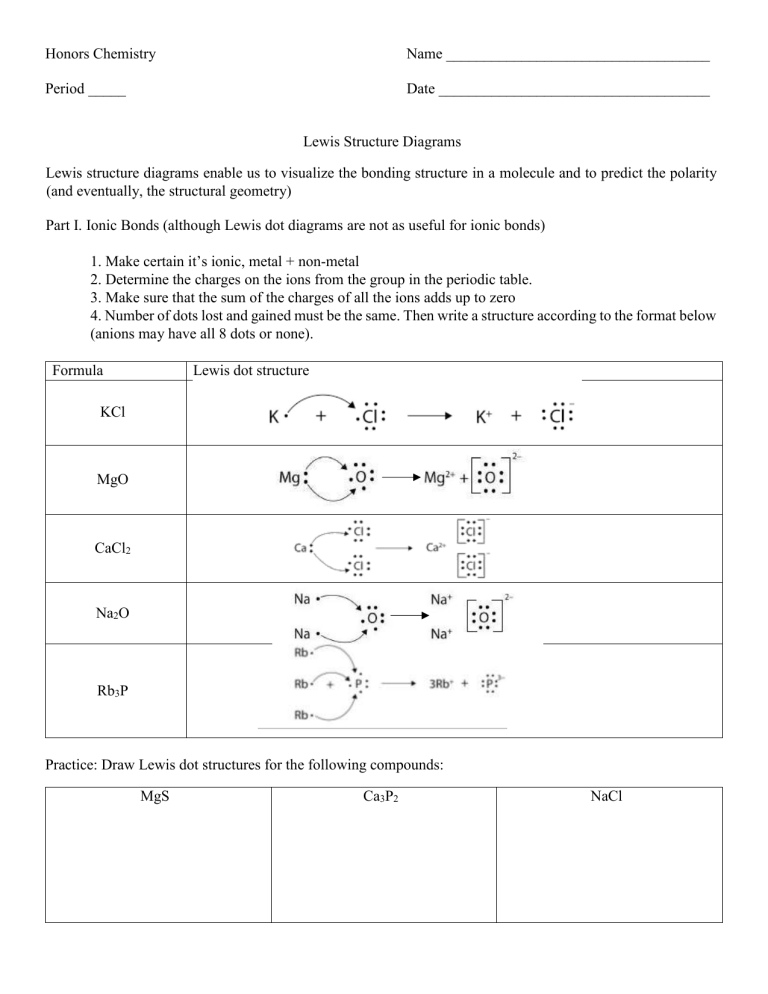
Honors Chemistry Name ___________________________________ Period _____ Date ____________________________________ Lewis Structure Diagrams Lewis structure diagrams enable us to visualize the bonding structure in a molecule and to predict the polarity (and eventually, the structural geometry) Part I. Ionic Bonds (although Lewis dot diagrams are not as useful for ionic bonds) 1. Make certain it’s ionic, metal + non-metal 2. Determine the charges on the ions from the group in the periodic table. 3. Make sure that the sum of the charges of all the ions adds up to zero 4. Number of dots lost and gained must be the same. Then write a structure according to the format below (anions may have all 8 dots or none). Formula Lewis dot structure KCl MgO CaCl2 Na2O Rb3P Practice: Draw Lewis dot structures for the following compounds: MgS Ca3P2 NaCl Fr2Se Al2O3 Na3N Li2O AlBr3 CaI2 Part II. Covalent Bonds. To construct a Lewis diagram for simple covalent compounds (with single bonds), follow these steps: 1. Look at the atoms that will be bonded. Choose the atom with the lowest electronegativity number to be the "central" atom (except that hydrogen cannot be a central atom). 2. Count up all of the valence electrons contributed by all of the atoms in the molecule. Divide by 2. This is the number of electron pairs available. 3. Place the remaining atoms around the central atom and put one pair of electrons (or a dash) between each of the surrounding atoms and the central atom. This is now the "skeletal" diagram. 4. Now use the remaining electron pairs to fulfill the octet for each atom and a duet for the hydrogen atoms. Electrons pairs not involved in bonding are "unshared pairs" and may contribute to polarity. If you do not have enough pairs to satisfy the octet, you may need to double up or triple up, creating double and triple bonds. Example: NH3 1. 2. 3. N is the central atom (H cannot be central) N has 5 valence electrons 5 valence electrons H has 1 valence electrons each = 3 valence electrons 8 valence electrons = 4 electron pairs H–N–H ׀ H 4. 3 electron pairs were used in the skeletal diagram. One unshared pair is left to fultill the octet for nitrogen. NH3 is polar due to the one pair of unshared electrons. ‥ H–N–H ׀ H For each of the following, indicate the number of valence electrons and draw the Lewis electron dot structure. HF NH3 CH4 NF3 Valence Electrons______ SiF4 Valence Electrons______ Valence Electrons______ F2 Valence Electrons______ Valence Electrons______ Cl2 Valence Electrons______ Valence Electrons______ H2 S Valence Electrons______ C2H6 Valence Electrons______ CCl4 Valence Electrons______ PF3 Valence Electrons______ C2F6 Valence Electrons______ Covalent Molecules with Multiple bonds If the molecule is covalent but the number of electrons does not allow all atoms to follow the octet rule (except hydrogen, of course), then the molecule probably has double and/or triple bonds. In this case, put pairs of electrons between atoms to make the multiple bonds. Usually you want to make sure the central atom has an octet first. Coordinate covalent bonds form when one atom donates a pair of electrons to share with another. Examples: CH2O Valence electrons: 12 N2 Valence Electrons______ HCN SO3 Valence electrons: 10 O2 Valence Electrons______ Valence electrons: 24 CO2 Valence Electrons______ SO2 Valence Electrons______ O3 C2H4 Valence Electrons______ Valence Electrons______ CH3CO2H Valence Electrons______ NO2 SiO2 Valence Electrons______ C2H2 Valence Electrons______ Valence Electrons______ ONF SCO Valence Electrons______ (put the N in between the O and F) Valence Electrons______ (put the C in between the S and O) Bonding in Polyatomic Ions Large ions in which atoms are covalently bonded together but often bond ionically with other atoms are called polyatomic ions. As a general rule, they do not make oxygen – oxygen bonds but radiate the oxygen atoms around the central atom, making coordinate covalent bonds if needed. Count the number of valence electrons for the atoms, then add or subtract negative charges as needed to give the correct final charge on the ion. Examples: NO3- NH4+ Valence electrons: 23 + 1 = 24 OH- Valence electrons: 9 – 1 = 8 Valence electrons: 8 NO2- ClO3- CO32- NO3- Valence Electrons______ Valence Electrons______ Valence Electrons______ Valence Electrons______ CN- PO3-3 SO42- PO43- Valence Electrons______ Valence Electrons______ Valence Electrons______ Valence Electrons______ For the following, give the name, draw the Lewis Structure, draw a three-dimensional representation using the principals of VSEPR, and show the polarity using the delta notation for partial positive and negative charges. SeCl2 Name: Lewis Structure NF3 Name: Lewis Structure ICl4Lewis Structure Name: PBr3 Name: Lewis Structure H3O+ Name: Lewis Structure AsCl5 Name: Lewis Structure BrF5 Lewis Structure Name: OF2 Name: Lewis Structure ClO3- Name: Lewis Structure TeF4 Lewis Structure Name:

