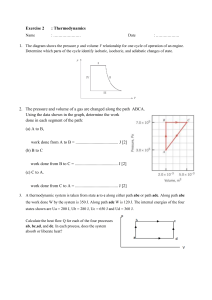
Name __________________________________ Date _________________
Thermodynamics AP
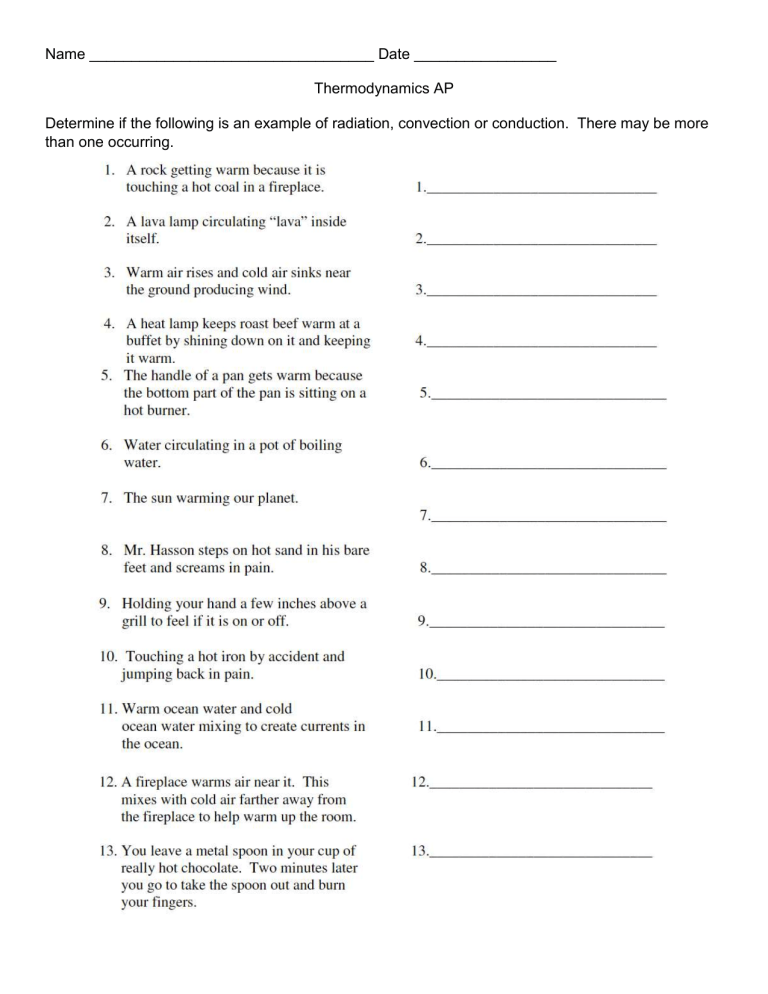
Determine if the following is an example of radiation, convection or conduction. There may be more than one occurring.
Name __________________________________ Date _________________
Thermodynamics AP
Temperature changes: 0K – 273.15 = ⁰C and (⁰F-32)x5/9=⁰C
Calculate the following
14. 100⁰C K ____________
15. 100K ⁰C ____________
16. 23⁰C K ____________
17. 97⁰F
18. 400K
Calculate the change in thermal energy. Use the following table
⁰C ____________
⁰C ____________
19. How much thermal energy needs to be added to 1.0 kg water to heat it from 22⁰C to 92⁰C?
____________
20.
How much thermal energy needs to be added to 0.455 kg benzene to heat it from 20.2°C to
31.4°C?
____________
Name __________________________________ Date _________________
Thermodynamics AP
21. Determine the heat lost when a)
3.7 kg of water cools from 31°C to 24°C b) a 540g piece of silver cools from 78°C to 14°C
____________
____________
22. An electric immersion heater delivers 0.050 MJ of energy to 5.0 kg of a liquid, changing its temperature from 32°C to 42°C. Find the specific heat capacity of the liquid.
____________
23. A 2.5kg pane of glass, initially at 41°C, loses 4.2 x 104 J of heat. What is the new temperature of the glass?
____________
24.
What is the new temperature of when 124 g of a liquid at 12.1°C is added to 0.375 kg of the same liquid a t 83.7°C?
____________
Name __________________________________ Date _________________
Thermodynamics AP
25.
Water has one of the highest values for specific heat capacities at 4186 J/kg°C, whereas sand has a much lower specific heat capacity at 290 J/kg°C. Compare the temperature increase for the given amounts of energy added.
26. Using the information above, provide a scientific explain why during the day the sand on a beach would be hotter than the nearby water, and why this reverses at night.
27
. A 3.25 g piece of gold is heated to 564°C. The gold is added to a cup containing 225 mL of water at 18.4°C. What is the final temperature of the gold and water after all thermal energy has transferred?
____________