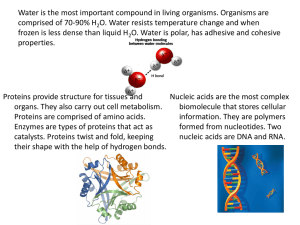
Chemicals or molecules present in living organisms Compounds of carbon The chemistry of living organisms is organized around carbon Building blocks Makes up biomolecules Carbohydrates – sugar Proteins – amino acid Nucleic acid – nucleotides Lipids – fatty acid and glycerol Carbohydrates – glycosidic bond Proteins – peptide bond Lipids – ester bond Nucleic acids – phosphodiester linkages Most abundant organic molecules in nature Defined as organic substances having C, H and O Wherein H and O are in the ratio of 2:1 Most abundant source of energy Precursors for many organic compounds Present as glycoproteins and glycolipids in the cell membrane and functions for cell growth and fertilization Present as structural components like cellulose in plants, exoskeleton of some insects, cell wall of microorganisms Storage form of energy (glycogen) to meet the energy demands of the body Carbohydrates Monosaccharide Oligosaccharide Polysaccharide BASED ON THE NUMBER OF C-ATOMS Triose (C3H6O3) Aldose Functional group is aldehyde Tetrose (C4H8O4) e.g. Glyceraldehyde Ketose Pentose (C5H10O5) Functional group is ketone Arabinose e.g. Dihydroxyacetone Hexose (C6H12O6) Glucose Glyceraldehyde Erythrose BASED ON FUNCTIONAL GROUPS Heptose (C7H14O7) Glucoheptose Formed by condensation of 2-9 monosaccharides Based on the number of monosaccharide molecules: Disaccharides – smallest and most common Trisaccharides Tetrasaccharides Consists of 2 monosaccharide units held together by a glycosidic bond Maltose Malt sugar Made up of two glucose molecules Lactose Milk sugar Made up of glucose and galactose Sucrose Cane sugar Made up of glucose and fructose Also called as glycans Made up of repeating units of monosaccharides held by glycosidic bonds Ideal as storage and as structural components 2 types: homoglycans and heteroglycans HOMOGLYCANS Made up of only 1 type of monosaccharide monomers E.g. starch, glycogen, cellulose Glucan (made up of glucose) Fructan (made up of fructose) Galactan (made up of galactose) HETEROGLYCANS Made up to 2 or more types of monosaccharides E.g. hyaluronic acid, agar, chitin, peptidoglycan STORAGE POLYSACCHARIDES Starch STRUCTURAL POLYSACCHARIDES Carbohydrate reserve of plants Most important dietary source for Occurs exclusively in plants Most abundant organic substance Predominant constituent of plant animals Amylose + Amylopectin Glycogen Cellulose cell wall Chitin Carbohydrate reserve of animals; Second most abundant organic referrred to as animal starch High concentration in liver, muscles and brain Glucose is the repeating unit substance Found in exoskeleton of some invertebrates like insects and crustaceans Provides strength and elasticity Becomes hard due to calcium carbonate Inulin Polymer of fructose Easily soluble in water Most abundant organic molecules of the living system Form about 50% of the dry weight of the cell Most important for the architecture and functioning of the cell Monomer – amino acids Collagen – most abundant animal protein Rubisco – most abundant plant protein Group of organic compounds having 2 functional groups (-NH2) and (–COOH) (-NH2) group is basic whereas (-COOH) is acidic R- can be H in glycine, CH3 in alanine, hydroxymethyl in serine, in others it can be a hydrocarbon chain or a cyclic group 4 basic structural levels are assigned to proteins Primary Secondary Tertiary Quaternary Primary – describes the unique order in which amino acids are linked together to form a protein Secondary – refers to the coiling or folding of a polypeptide chain that gives the protein its 3-D shape Tertiary – refers to the comprehensive 3-D structure of the polypeptide chain of a protein Quaternary – refers to the structure of a protein macromolecule formed by interactions between multiple polypeptide chains Structural proteins Enzymatic proteins Transport proteins Hormonal proteins Functional Classification Contractile proteins Storage proteins Genetic proteins Defense proteins Receptor proteins Simple proteins Composed only of amino acid residues Conjugated proteins Along with amino acids, there is a non-protein prosthetic group Derived proteins Denatured or degraded products Chief concentrated storage form of energy forming about 3.5% of the cell content Hydrophobic (“water-fearing”) or insoluble in water, because they are Concentrated fuel reserve of the body Constituents of membrane structure and regulate membrane permeability Serve as source of fat soluble vitamins Important cellular metabolic regulators Protect the internal organs and serve as insulating materials Made up of fatty acids and glycerol Fatty acids may be saturated or unsaturated Saturated fatty acids – solid at room temp Unsaturated fatty acids – liquid at room temp Monomer - nucleotides 2 types: DNA (deoxyribonucleic acid) RNA (ribonucleic acid) Storage for genetic information Sequence of nitrogenous bases in DNA determines the protein development in new cells DNA controls the synthesis of RNA The purines adenine (A) and guanine (G) and the pyrimidine cytosine (C) are present in both DNA and RNA. The pyrimidine thymine (T) present in DNA is replaced by the pyrimidine uracil (U) in RNA.