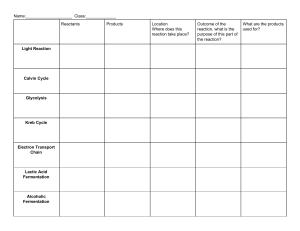
GENERAL BIOLOGY I Laboratory Activity: FERMENTATION Fermentation is the anaerobic oxidation of sugars to form ethanol or ethyl alcohol. On the other hand, aerobic oxidation of sugars will produce acetic acid. Wine preparation is not limited only to the fermentation of sugar cane. It can also be done to any kind of fruit that may be in season during the performance of the experiment, as long as the amount of sugar is adjusted to provide enough, substrate for the production of alcohol. Yeast (Saccharomyces cerevisiae), is a fungi that produces the enzyme to hasten the anaerobic/aerobic oxidation of the sugar. Brewer’s yeast is more preferred that Baker’s yeast but the latter is used in the experiment due to economic considerations, and the availability of the material. OBJECTIVES: At the end of the experiment, the student will know how to: 1. Prepare wine from indigenous materials. 2. Utilize different type of fruits to produce acetic acid or vinegar. EQUIPMENT AND MATERIALS: 1. 2 Fermentation jars 2. Cheese (cotton) cloth (as strainer) 3. Measuring cups 4. Yeast 5. Fruits 6. Brown sugar/white sugar 7. Thermometer PROCEDURE: 8. Cooking Pot for Pasteurization 9. 2L Distilled Water 10. Knife 11. Cutting Board 12. Bowl 13. Stirring Rod/ Spatula A. Wine Preparation 1. Wash and peel ripe (or slightly overripe) fruits. 2. Crush or grate the peeled fruits to extract the juice. 3. Alternatively, use blender to fully extract the juice of the fruit. 4. Dilute the crushed fruit extract with the proportion, 1 cup of water to 1 cup of the juice. 5. Add 1 cup of sugar to every 4 cups of the diluted juice. Mix thoroughly and pasteurize through heat over a low flame for 30 minutes. Alternatively, add more sugar to give variation on the flavor of wine. Note: With higher sugar concentration, the longer the fermentation process. 6. Cool and pour the mixture into a bottle (fermenting container) three-fourths (3/4) full. 7. Add ½ teaspoon of dry yeast to every litter of the mixture. 8. Stopper with a cork, fitted with a U-tube in which the other end is dipped in a test tube filled with Calcium hydroxide solution. 9. Alternatively, use condom to stopper the mouth of the fermenting jar. This will allow you to monitor the amount of CO2 gas produced during the fermentation. 10. Allow the mixture to ferment for three to four weeks or until no more bubbles are formed, or until the latex balloon stopped inflating. 11. After fermentation, strain and pasteurize the prepared wine at 65℃ for 30 minutes. 12. If impurities are observed, let the particulate settle and decant in a suitable sterilized container and pasteurize 13. Alternatively, to make the wine clear, add a well-beaten egg white to every 12 cups of the mixture, then pasteurize. 14. Let the wine cool down. Stopper tightly and label. B. Vinegar Preparation 1. Alcoholic Fermentation a. Dissolve one (1) part of sugar to eight (8) parts of the fruit juice extract. b. Filter through cheese cloth and pasteurize for 20 minutes. c. Cool to room temperature and pour into the fermentation bottle, ¾ full. d. Add 1 teaspoonful of yeast to every 1-liter bottle. e. Protect the fermenting mass from dust by covering the mouth of the bottle with clean cloth. f. Allow to ferment for 4-7 days or until an appreciable characteristic odor is observed. 2. Oxidation of Alcohol a. Add one (1) part mother vinegar to eight (8) parts of the alcoholic solution prepared above. b. Filter and set aside for two (2) weeks or until the desired acidity is obtained. c. Pasteurize. EVALUATION Wine Preparation Guide Questions: 1. What type of respiration is employed in the process of wine making? 2. How is alcohol produced through this process? Specify the reaction process by writing the complete and balanced chemical equation. 3. Yeast (Saccharomyces cerevisiae) has been used for food and beverage preparation for hundreds of years. Explain the importance and the mechanism of these microorganism in producing such products. 4. Observe the change in appearance of the solution. What do you think is the reason behind this change in appearance along the process of fermentation? 5. How about the odor and texture? Explain how the fermentation process affects the change in odor and texture of the solution. 6. Is there any change on the pH Level of the solution? Explain what causes this change in pH level of the solution. Observations: PARAMETERS Color/Appearance of the Solution Odor Texture pH Level OBSERVATIONS Initial Final Vinegar Preparation Guide Questions: 1. What type of respiration is employed in the process of vinegar making? 2. Specify what compound is produced in this reaction by writing the complete and balanced chemical equation of this process. 3. The mechanism in the production of vinegar is quite different with that of the wine production using microorganisms. Give an explanation on the mechanism on how these microorganisms enhance the reaction thereby producing the desired products. 4. Observe the change in appearance of the solution. What do you think is the reason behind this change in appearance along the process of fermentation? 5. How about the odor and texture? Explain how the fermentation process affects the change in odor and texture of the solution. 6. Is there any change on the pH Level of the solution? Explain what causes this change in pH level of the solution. Take note of the pH level for 4 (four) observation periods with 3 replicates. 7. Use Analysis of Variance (ANOVA) specifically Completely Randomized Design at 5% significance level, as Statistical Analysis for the comparison of the pH Level for each of the observation periods. 8. Is their significance difference between the observation periods? Show your Table of Means and give a graphical illustration of the result. Discuss the result of the experiment. 9. What is the implication of these result? 10. Include documentations in the appendices for the methods and results. Observations: OBSERVATIONS PARAMETERS 1 2 3 4 Color/Appearance of the Solution Odor Texture R1 R2 R3 R1 R2 R3 R1 R2 R3 R1 R2 R3 pH Level Summary, Conclusion and Recommendations: . *Use extra sheet for your lab results. *Use only long sized bond paper for your outputs. INDIVIDUAL PERFORMANCE EVALUATION Group Member Names Name of Rater Asst. Surgeon: Workload Contribution(s) Rating A– B– C– D– E– TOTAL – Chief Surgeon: * Rating of Group Members to be accomplished by the Chief Surgeon. * Rating of the Chief Surgeon to be accomplished by anyone of the Assistant Surgeon. * Refer to the attached Rubric for the pointing of the members. * Adjust/Add rows if necessary. RUBRIC IN LABORATORY ACTIVITY FOR GENERAL BIOLOGY Criteria (A) Set-up and Equipment Care (B) Following Procedure (C) Data Collection (D) Safety (E) Clean-up 1 Set-up of equipment is not accurate, help is required with several major details Many necessary supplies must found in mid-lab 2 Set-up of equipment is generally workable with several details that need refinement Some necessary supplies must be searched out 3 Set-up of equipment is generally accurate with 1 or 2 small details that need refinement All necessary supplies on hand 4 All equipment accurately placed All necessary supplies on hand Demonstrates good knowledge of the Demonstrates sound Lacks the appropriate lab procedures knowledge of lab Demonstrates knowledge of the Will ask peers for procedures general knowledge lab procedures help with Will discuss with of lab procedures Often requires help problems in lab peers to solve Requires help from from the teacher to procedures problems in teacher with some even complete Works to follow each procedures steps in procedures basic procedures step before Carefully follows moving on to the each step next step Measurements are incomplete, inaccurate and imprecise Observations are incomplete or not included Symbols, units and significant figures are not included Measurements are somewhat inaccurate and very imprecise Observations are incomplete or recorded in a confusing way There are 3 or more minor errors using symbols, units and significant digits or 2 major errors Measurements are Measurements are accurate with mostly accurate reasonable Observations are precision generally complete Observations are Work is organized thorough Only 2 or 3 minor Work is generally errors using neat and organized symbols, units and Includes symbols, significant digits units and significant digits Proper safety precautions are consistently missed Needs to be reminded often during the lab Proper safety precautions are often missed Needs to be reminded more than once during the lab Proper safety precautions are generally used May need to be reminded once during the lab Proper safety procedures are consistently used Uses general reminders of safe practices independently Proper clean-up procedures generally used May need some help on occasion to complete tasks Station generally left clean Consistently uses proper clean-up procedures Station generally neat and clean Proper clean-up Needs to be procedures are reminded more seldom used than once during Often requires help the lab to use to complete cleanproper clean-up up procedures 3 or more items left 1 or 2 items left at at station or station station or not not cleaned cleaned 5 All equipment accurately placed All necessary supplies on hand Very neat and organized Demonstrates very good knowledge of the lab procedures Gladly helps other students to follow procedures Thoroughly and carefully follows each step before moving on to next step Measurements are both accurate and precise Observations are very thorough and may recognize possible errors in data collection Work is neat and organized Includes appropriate symbols, units and significant digits Proper safety precautions are consistently used Consistently thinks ahead to ensure safety Will often help other students to conduct labs safely Consistently uses proper clean-up procedures Often will help other students to complete tasks properly Station always left neat and clean