Uploaded by
Mahmoud Mahrous
Thermodynamics Lecture Notes: Laws, Systems, and Equations
advertisement
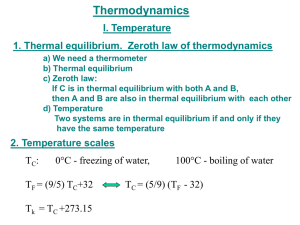
MSE 202 2019F Lecture #1 page 1 Thermodynamics Thermodynamics: Describes ___________ properties of ___________ systems Entirely ___________ Built on ___________ and mathematics 0th Law Defines ____________________ 1st Law Defines ____________________ 2nd Law Defines ____________________ 3rd Law Gives ____________________ These laws are ____________________, they ___________ be circumvented. Lecture #1 Definitions: ● System: ____________________ ● Surroundings: ____________________ ● Boundary: ____________________ Systems can be: ● Open: ____________________ can transfer between the ____________________ ● Closed: ____________________ can transfer between the ____________________, but NOT ____________________ ● Isolated: ____________________ can transfer between the ____________________ Describing systems requires: ● A few macroscopic properties: ____________________ ● Knowledge if System is ____________________ ● Knowledge if System is in ____________________ ● Knowledge of ____________________ page 2 Lecture #1 page 3 Two classes of Properties: • Extensive: ________________________ ______________________ • Intensive: ________________________ _________________ The State of a System at Equilibrium: • Defined by the collection of all macroscopic properties that are described by ________________________ [______________________________of the SYSTEM] • For a one-component System, all that is required is ____________________. All other properties then follow. V = f (_____) or p = g(_____) • Notation: 3 moles (n=3) gas 2 Cl2 (g, 5 L, 50 o C) p=1 bar , T=100 oC 5 Ar (s, 5 bar, 50 K) Lecture #1 Change of State: (Transformations) • Notation: 3 H2 (g, 5 bar, 100 oC) = 3 H2 (g, 1 bar, 50 o C) _____ state _____ state • Path: Sequence of intermediate states 5 p (bar) 1 50 100 T (K) • Process: Describes the Path - Reversible - Irreversible - Adiabatic ______________________ ______________________ ______________________ ______________________ _____________________ _____________________ - Isobaric - Isothermal - Constant Volume - page 4 Lecture #1 page 5 Thermal Equilibrium (heat stops flowing) A B A B A B When a hot object is placed in thermal contact with a cold object, heat flows from the warmer to the cooler object. This continues until they are in thermal equilibrium (the heat flow stops). At this point, both bodies are said to have the same “____________”. This intuitively straightforward idea is formalized in the __________________ _______________ and is made practical through the development of thermometers and temperature scales. ===== ZERO’th LAW of Thermodynamics ===== If then A and B are in thermal equilibrium and B and C are in thermal equilibrium, A and C are in thermal equilibrium. Consequence of the zero’th law: B acts like a ____________, and at the same “____________”. A , B , and C are all Lecture #1 page 6 Operational definition of temperature (t) Need: (1) __________________ (2) __________________ (3) __________________ (4) __________________ Example: Ideal Gas Thermometer with the Celsius scale. Based on ____________________ lim (pV )= constant = f (t ) p →0 t depends on t for fixed t • the substance is a __________________ • f (t ) is the _________ • the __________________ and __________________ of water are the reference points • the interpolation is __________________ p 0 f(t)=f(0 oC)(1+At) Experimental result: A = _________=_________ -273.15 Note: 0 100 C (t = −273.15 °C ) is special t = −273.15 °C is called _______________ Lecture #1 ======= This suggests defining ____________________________ T (K ) = __________________ T = 0K corresponds to _____________________ ======= Better reference points used for the Kelvin scale today are T = ____________and Ttp = _______________________ p 0 f(Ttp) 0 0 T(K) ______= Ttp page 7 Lecture #1 page 8 Ideal Gases Boyle’s Law and the Kelvin scale valid for all gases for ________ An ideal gas obeys the expression _________at all pressures (the gas molecules ____________________) The Ideal gas law or This is an example of an equation of state __________________________ Lecture #1 page 9 Equations of state IDEAL GAS LAW: Mixture of ideal gases comprising ni moles of each Partial pressure of ith gas mole fraction of i th gas Dalton’s Law Real Gases (a) (b) -- do not necessarily obey ideal gas law Compressibility factor High T ⇒ __________ dominate Low T _________ dominate ⇒ Virial Expansion generally neglect B = 0 ideal gas (neglect C and higher order terms) Lecture #1 (c) van der Waals Equation of state only two parameters, derived from molecular concepts • First assume “hard sphere” molecules becomes • Now put in attraction So Rearranging becomes page 10