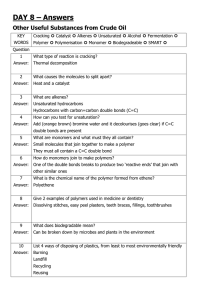
Degree of Unsaturation with Iodine & Saponification of Fatty Acids Abstract In this lab different oils including sunflower oil, castor oil, grapes oil, avocado oil and unknown oil were tested for their degree of unsaturation using iodine and their soap characteristics were determined using sodium hydroxide. It was found that, grape seed oil has highest degree of unsaturation, whereas, sunflower has least. The decreasing order of unsaturation is grape seed oil < unknown oil < castor oil < avocado oil < sunflower oil. Moreover, the soap formation characteristic of the oils in increasing order is Sunflower oil < Avocado oil < unknown oil < castor oil < grape seed oil. The effect of hard water and soft water on soap formation was also determined. When soft water was added to the oil, it was found that hand soap oil is most sudsy and sunflower is most least sudsy. However, when Magnesium sulfate was added to each vial no effect was measured (it could be error) because magnesium sulfate should reduce the soapy nature of the oils. Introduction An unsaturated hydrocarbon is a molecule made of carbon and hydrogen atoms that does not contain the maximum number of hydrogens possible. An alkene is an unsaturated hydrocarbon. The general formula for calculating the degree of unsaturation is: degree of unsaturation = C – H/2 - X/2 + N/2 + 1 Using this equation, C represents the number of carbon atoms in the molecule, H represents the number of hydrogen atoms, X represents the number of halogen atoms, and N represents the number nitrogen atoms. Cooking oils are a common example of fatty acids. Some cooking oils are saturated fatty acids, and some are unsaturated. The difference is a double bond. All fatty acids have a carboxylic acid on one end of the molecule. In this lab different oils including sunflower oil, castor oil, grapes oil, avocado oil and unknown oil were tested for their degree of unsaturation using iodine and their soap characteristics were determined using sodium hydroxide Procedure As given in the lab manual. Results Addition of iodine on oils 2. order of degree of unsaturation The decreasing order of unsaturation is grape seed oil < unknown oil < castor oil < avocado oil < sunflower oil. 3. Effect of Sodium Hydroxide and Soft water in oils 4. Effect of MgSO4 in oils 5. order of Least Sudsy to Most Sudsy oil Discussion and Conclusion Sunflower oil, castor oil, grapes oil, avocado oil and unknown oil were tested for their degree of unsaturation using iodine and their soap characteristics were determined using sodium hydroxide. It was found that, grape seed oil has highest degree of unsaturation, whereas, sunflower has least. The decreasing order of unsaturation is grape seed oil < unknown oil < castor oil < avocado oil < sunflower oil. The literature says grape seed oil contains more than 80 % unsaturated compounds, out of them 70 % linolenic acid and 16 % oleic acid. Grape seed oil contains 80% of poly unsaturated fatty acids. The reaction of linolenic acid with iodine is as following; Castor oil contains 85-95% ricinoleic acid is the main component. Avocado oil contains 13.5% total polyunsaturated fatty acids and 71 % monounsaturated fatty acids. linoleic acetogenin Sunflower oil contains 3.8 % total polyunsaturated fatty acids and 83% monounsaturated fatty acids. The structure is given in the following; Moreover, Soap characteristic of oils was also determined and observed increasing order of soapy nature of the oils, Sunflower oil < Avocado oil < unknown oil < castor oil < grape seed oil. So, based on the literature, we conclude that grape seed contains highest percentage of poly unsaturated fatty acids and therefore, it showed greater extent of soap formation characteristic and sunflower has least due to less polyunsaturated fatty acid chains. The saponification reaction is given as following.