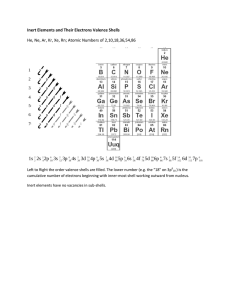
Name:______________________________________ Date:____________ Class Period:_______
Periodic Table Quiz 1
1.
What type of element becomes a 5.
What are valence electrons? cation? a.
electrons in the outer shell of a.
Metals b.
Nonmetals c.
Period 2 elements d.
All of the above
2.
Which of the following groups is most likely to lose 2 electrons to become stable? b.
c.
d.
an atom electrons in the inner shell of the atom core electrons
All of the above
6.
Which of the follow statements best a.
Alkali Metals, because they will have a full outer shell if they lose 2 electrons b.
Alkaline Earth Metals, describe Noble Gases? a.
They are highly reactive. b.
They have a full outer shell and do not react with other because they have 2 electrons in their outer shell elements. c.
They have 18 valence electrons. c.
Halogens because they only need 2 more electrons to be stable d.
None of the above.
3.
Based on your knowledge of the periodic table, how many electron d.
None of the above.
7.
What are the two main sections of the Periodic Table? a.
b.
Liquids and Gases
Metals and Metalloids c.
Metals and Nonmetals d.
All of the above
8.
Which of the following statements is shells do the elements in period 3 have? a.
Each element has a different amount of electron shells. b.
All of the elements in a period have the same amount true about alkaline earth metals? a.
They generally form cation because they have 2 valence electrons. c.
d.
of electrons shells. All the elements in period 3 have 3 electron shells.
It depends on how many electrons they have. d.
All of the above.
4.
What is an ion? a.
an element that has lost or gained protons b.
a neutral element c.
an element that has lost or gained electrons. an element with different amounts of neutrons b.
They will lose electrons to achieve a full outer shell. c.
They are very reactive. d.
All of the above
9.
Which group's elements have one electron in the outer shell? a.
Alkali Metals b.
Transition metals c.
Noble Gases d.
Alkali Earth Metals
10.
How many electrons does the element Te have in its outer shell? * a.
1 b.
16 c.
15 d.
6
Name:______________________________________ Date:____________ Class Period:_______
Periodic Table Quiz 1
11.
How many electron shells does 17.
In an ion, the atomic number is the
Chlorine (Cl) have? same as the _____________. a.
3 b.
17 c.
7 d.
4 a.
number of protons b.
number of electrons c.
number of neutrons d.
the atomic mass
Consider the ion: Cl 1Consider Zinc.
12.
How many PROTONS? a.
18 b.
17 c.
9 d.
10
13.
How many ELECTRONS? a.
8 b.
16 c.
17 d.
18
14.
Is it a cation or anion? a.
Cation b.
Anion
15.
How many electrons are lost or gained? a.
Gained 1 b.
Lost 1 c.
Gained 2 d.
Lost 2
16.
All of the following true about
PERIODS on the periodic table
EXCEPT: a.
All elements in a period have the same number of electron shells. b.
The chemical and physical properties of the elements within a period are very different. c.
The atomic mass of elements increase as you move across(left to right) a period. d.
All the elements in a period have the same number of valence electrons.
18.
How many PROTONS? a.
30 b.
65 c.
35 d.
67
19.
How many NEUTRONS? a.
30 b.
65 c.
35 d.
67
20.
How many ELECTRONS? a.
30 b.
65 c.
35 d.
67