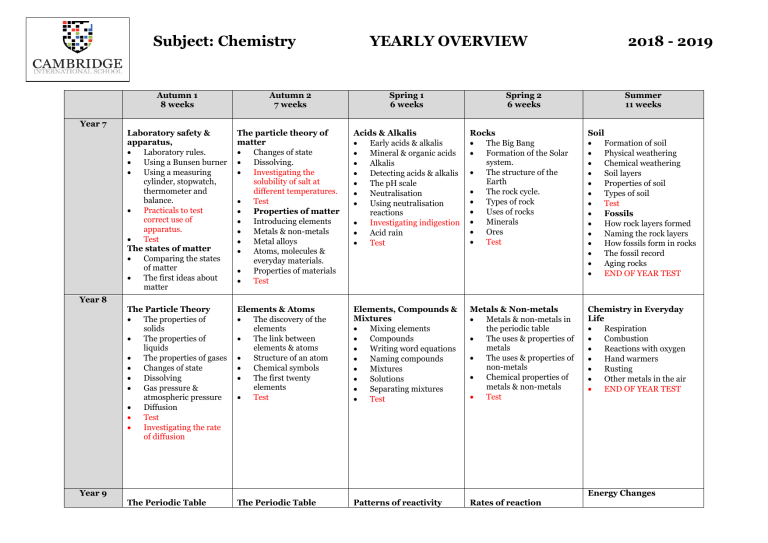
Subject: Chemistry Year 7 YEARLY OVERVIEW Spring 2 6 weeks 2018 - 2019 Autumn 1 8 weeks Autumn 2 7 weeks Spring 1 6 weeks Summer 11 weeks Laboratory safety & apparatus, • Laboratory rules. • Using a Bunsen burner • Using a measuring cylinder, stopwatch, thermometer and balance. • Practicals to test correct use of apparatus. • Test The states of matter • Comparing the states of matter • The first ideas about matter The particle theory of matter • Changes of state • Dissolving. • Investigating the solubility of salt at different temperatures. • Test • Properties of matter • Introducing elements • Metals & non-metals • Metal alloys • Atoms, molecules & everyday materials. • Properties of materials • Test Acids & Alkalis • Early acids & alkalis • Mineral & organic acids • Alkalis • Detecting acids & alkalis • The pH scale • Neutralisation • Using neutralisation reactions • Investigating indigestion • Acid rain • Test Rocks • The Big Bang • Formation of the Solar system. • The structure of the Earth • The rock cycle. • Types of rock • Uses of rocks • Minerals • Ores • Test Soil • Formation of soil • Physical weathering • Chemical weathering • Soil layers • Properties of soil • Types of soil • Test • Fossils • How rock layers formed • Naming the rock layers • How fossils form in rocks • The fossil record • Aging rocks • END OF YEAR TEST The Particle Theory • The properties of solids • The properties of liquids • The properties of gases • Changes of state • Dissolving • Gas pressure & atmospheric pressure • Diffusion • Test • Investigating the rate of diffusion Elements & Atoms • The discovery of the elements • The link between elements & atoms • Structure of an atom • Chemical symbols • The first twenty elements • Test Elements, Compounds & Mixtures • Mixing elements • Compounds • Writing word equations • Naming compounds • Mixtures • Solutions • Separating mixtures • Test Metals & Non-metals • Metals & non-metals in the periodic table • The uses & properties of metals • The uses & properties of non-metals • Chemical properties of metals & non-metals • Test Chemistry in Everyday Life • Respiration • Combustion • Reactions with oxygen • Hand warmers • Rusting • Other metals in the air • END OF YEAR TEST The Periodic Table The Periodic Table Patterns of reactivity Rates of reaction Year 8 Year 9 Energy Changes • • • • • • • • • • Chemical symbols review How the elements are organised Sub-atomic particles Electron arrangement of first twenty elements. Which elements react with which Loss or gain of electrons to form ions Writing a chemical formula Balancing chemical equations Group I – the alkali metals Test (Contd) • Group II – the alkaline earth metals • Group VII – the halogens • Group 0 – the noble gases • Hydrogen • Periodic trends • Test Preparing common salts • Word & chemical symbol equations for making salts • Acids & their salts • Preparing a salt from a metal & an acid • Preparing a salt from a metal carbonate & an acid • Test • • • • • • How metals react with oxygen How metals react with water How metals react with acids Displacement reactions The reactivity series Test • • • • • Measuring rate of reaction Factors affecting rates of reaction The particle theory and rates of reaction Test • • • • Exothermic & endothermic reactions Energy considerations for physical changes & chemical reactions Measuring energy in a fuel Examples of exothermic & endothermic reactions END OF YEAR TEST




