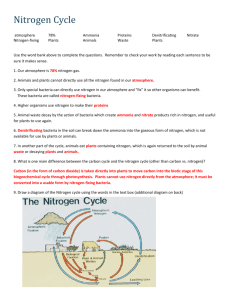
Cycles Cheat Sheet Water Cycle Precipitation -> Runoff -> Evaporation-> Transpiration-> Condensation Sun powers evaporation, precipitation, and transpiration Reservoirs – Surface water (oceans, rivers, lakes), Glaciers, Groundwater (Aquifers), Water Vapor Atmospheric Gas, Living Organisms Importance Water is essential to living organisms for a variety of reactions and cell processes Water’s high heat storage distribute heat and determine regional and local climates Water is a greenhouse gas – essential for maintaining Earth’s temperature – BUT too much greenhouse gas can be a bad thing Sculpts landscapes It is the universal solvent, often interacts with the movement of other cycles because it transports them The water cycle is nature’s natural water purifier (evaporation and underground bacteria) Water is a necessity in industry, waste management How humans alter Overuse – use faster than it can replenish Increase pollutants in runoff Reduce infiltration – hard paved surfaces prevent recharging of groundwater Accelerate topsoil erosion Increase risk of flooding – when we drain and fill wetlands for farming or urban development Alter weather – deforestation reduces transpiration of rainforests, the primary source of rainfall in rainforests. Reduced shade also evaporates water before it can permeate the soil. Carbon Cycle Found in the atmosphere as a gas (CO2), dissolvable in water, Producers pull carbon from the atmosphere or water for the process of photosynthesis Consumers take in carbon by ingesting producers or other consumers Producers and consumers release carbon as a product of respiration Carbon can be incorporated into marine and terrestrial sediments After millions of years carbon can be compressed into fossil fuels Reservoirs – the ocean is the largest reservoir, calcium carbonate found in shells, marine sediments (decomposers release insoluble carbonates from dead marine organisms) forming limestone as well, fossil fuels (oil, natural gas, coal), tropical rain forests and “old growth” forests (those whose trees are hundreds to thousands of years old), tundra (permafrost), atmosphere, living organisms Importance Carbon is the basic building block of the four major macromolecules: carbs, lipids, proteins, DNA, as well as other important organic molecules. Carbon dioxide is a greenhouse gas – essential for maintaining Earth’s temperature – BUT too much greenhouse gas can be a bad thing How humans alter The extraction and burning of huge quantities of fossil fuels that have taken millions of years to form have released large amounts of carbon dioxide to the atmosphere The clear-cutting of carbon-absorbing vegetation (especially tropical rain forests) have released large amounts of carbon to the atmosphere The increased concentration of carbon dioxide and other greenhouse gases (methane, water) are warming our planet and projected to change Earth’s climate this century Nitrogen Cycle N2 cannot readily be absorbed and used by plants and animals, so we rely on electrical discharges (lightening) and nitrogen fixing bacteria to make it usable for plants Animals consume plants or other consumers to obtain their nitrogen Reservoirs – the atmosphere (78%) Remember “FNAAD, ANPAN” Fixation-------------- Ammonia Nitrification---------- Nitrates, Nitrites Assimilation---------- Proteins (Bacteria and cyanobacteria combine gaseous N2 with hydrogen to make ammonia NH3 and/or ammonia ions NH4+ which they use as a nutrient for themselves. They excrete the rest of the ammonia into the soil or water) (Ammonia not taken up by plants is converted by soil bacteria into nitrate ions NO3- which are easily taken up by the roots of plants) (Plants use the ammonia or nitrate ions to produce proteins, nucleic acids, and vitamins. Consumers will ingest plants or other consumers and do the same) ----Organism Death/Decomposition----------------------------------------------------------------------------------------------------------------------------------------------------------------- Ammonification------ Ammonia Denitrification-------- Nitrogen Gas (Decomposer bacteria convert detritus material into ammonia NH3 and ammonia ions NH4+) (Specialized bacteria in waterlogged soil and the bottoms of lakes, oceans, swamps, and bogs covert ammonia NH3 and ammonia ions NH4+ back into nitrate ions and then into nitrogen gas and nitrous oxide gas. These are released back to the atmosphere to begin the cycle again.) Importance Nitrogen is a key component in macromolecules such as proteins, vitamins, and nucleic acids (DNA and RNA) Nitrogen is a limiting factor for primary productivity (without it plants can’t grow!) Human Impacts We add nitric oxide NO to the atmosphere as we burn fuel at high temperatures (cars, jets). This gas is converted into nitrogen dioxide gas NO2 and nitric acid vapor HNO3 which falls to the earth as acid deposition aka acid rain. Anaerobic bacteria break down inorganic fertilizer or organic manure and contribute nitrous oxide N2O to the atmosphere. Nitrous oxide is a greenhouse gas. Deforestation releases large quantities of nitrogen stored in soils and plants, back to a gas Agricultural runoff of fertilizers, animal manure, and sewage discharge add excess nitrates NO3- to bodies of water. This can lead to eutrophication (algal blooms, which bring in more bacteria as algal decomposes, decreasing the oxygen in aquatic environment, often resulting in fish kills) Harvesting of crops, irrigation, and burning or clearing of grasslands and forests can cause nitrogen to wash away from the topsoil. We have more than doubled the annual release of nitrogen from the land due to the inorganic fertilizers Phosphorus Cycle Very slow to cycle in comparison to water, carbon, and nitrogen Water erodes away inorganic compounds, that include phosphates, from rocks Water carries dissolved phosphates into the soil where they can be absorbed by producers Consumers take in phosphates by ingesting producers Phosphates can be lost from the cycle for long periods when it is washed to the ocean and held in the marine sediment for millions of years Reservoirs – Phosphate salts containing phosphate ions, terrestrial rock formations, and ocean bottom sediments. Does not include the atmosphere Importance Phosphate ions PO43- are an important nutrient Phosphate is a limiting factor, particularly in aquatic systems, as it does not easily cycle (no gaseous state) and can take a long time to enter aquatic biomes. Therefore is limiting factor for plant growth! Phosphate is incorporated into nucleic acids, ADP, and ATP, bones, and teeth Human Impacts Humans mine phosphate salts which are added to fertilizers and applied to agricultural fields. Excess phosphates from runoff can also cause eutrophicaton (algal blooms, which bring in more bacteria as algal decomposes, decreasing the oxygen in aquatic environment, often resulting in fish kills) Clearing of rain forests causes phosphates to wash away