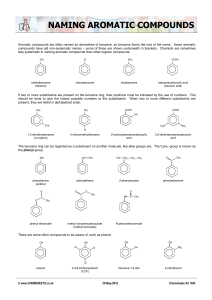
A2 4 39 © RWGrime (Chemsheets.co.uk) 24/10/19 4.6 – NAMING AROMATIC COMPOUNDS Aromatic compounds are often named as derivatives of benzene, so benzene forms the root of the name. Some aromatic compounds have old non-systematic names – some of these are shown underneath. However, chemists seem to deviate and break the rules when it comes to naming aromatic compounds, and at A level the crucial thing is to be able to work out the structure from the name. CH3 Cl methylbenzene (toluene) NO2 chlorobenzene COOH nitrobenzene benzenecarboxylic acid (benzoic acid) If two or more substituents are present on the benzene ring, their positions must be indicated by the use of numbers. This should be done to give the lowest possible numbers to the substituents. When two or more different substituents are present, they are listed in alphabetical order. COOH CH3 CH3 COOH OH CH3 O2N NO2 Cl 1,3-dimethylbenzene (m-xylene) 4-methyl-1-chlorobenzene 2-hydroxybenzenecarboxylic acid 3,5-dinitrobenzenecarboxylic acid The bezene ring can be regarded as a substituent on another molecule, like alkyl groups are. The C 6H5- group is known as the phenyl group. NH2 CH CH2 CH3 CH2 CH2 CH3 O CH3 C phenylamine (aniline) phenylethene O O O C phenylethanone O CH3 C O 2-phenylbutane NH C CH3 CH3 phenyl ethanoate methyl benzenecarboxylate (methyl benzoate) N-phenylethanamide There are some other compounds to be aware of, such as phenol. OH OH OH Cl Cl OH OH NO2 Cl phenol 2,4,6-trichlorophenol (TCP) benzene-1,2-diol 2-nitrophenol