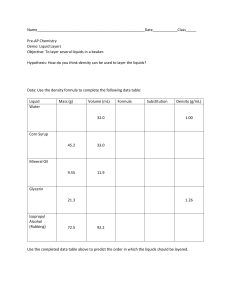
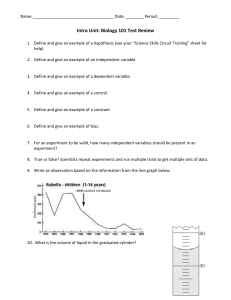
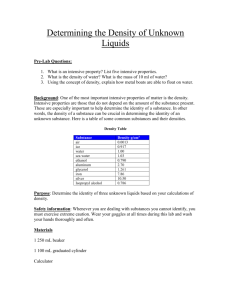
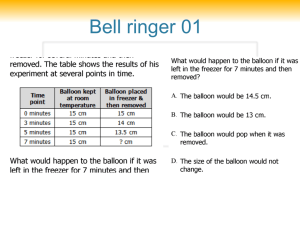
DENSITY LAB NAME_______________________________________________ PERIOD_____________ PROBLEM: HOW DO DENSITIES OF LIQUIDS AND OBJECTS DIFFER? MATERIALS: BEAKER, GRADUATED CYLINDER, CLEAR CUP, VARIOUS ITEMS TO TEST DENSITY, WATER, ALCOHOL, OIL, FOOD COLORING PROCEDURE 1. MEASURE 100 MLS USING THE GRADUATED CYLINDER INTO THE CLEAR CUP OF THE FOLLOWING: OIL, WATER, AND ALCOHOL 2. DROP TWO DROPS OF BLUE FOOD COLORING IN WATER, TWO DROPS OF GREEN FOOD COLORING IN ALCOHOL 3. POUR THE THREE LIQUIDS INTO A BEAKER IN THIS ORDER: WATER, OIL, ALCOHOL 4. CHOOSE A CARTON OF OBJECTS, LIST OBJECTS ON PAPER. 5. PREDICT WHERE IN THE COLUMN OF LIQUIDS THE OBJECT WILL FLOAT 6. DROP EACH OBJECT INTO THE LIQUID COLUMN, RECORD YOUR OUTCOME 7. WHEN FINISHED DRAIN THE CONTENTS OF YOUR BEAKER INTO THE BUCKET USING A PIECE OF NETTING TO CATCH OBJECTS. RETURN OBJECTS TO EGG CARTON. OUTCOME – OF THE LIQUIDS, ALCOCHOL HAD THE LEAST DENSITY, THEN OIL. WATER HAD THE MOST DENSITY. OBJECTS GREATER THAN 1 CUBIC GRAM SANK TO BOTTOM. OBJECTS LESS THAN 1 CUBIC GRAM FLOATED ON WATER. DENSITY LAB NAME_______________________________________________ PERIOD_____________ OBJECT PREDICTION OUTCOME Bean oil bottom Wire alchohol bottom Straw alchohol oil Bolt bottom bottom String alchohol alchohol Bobber alchohol alchohol Draw the beaker and its layers, list each object in the layer.