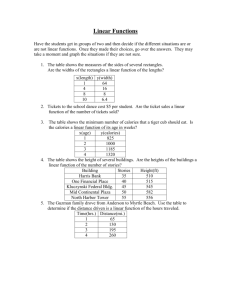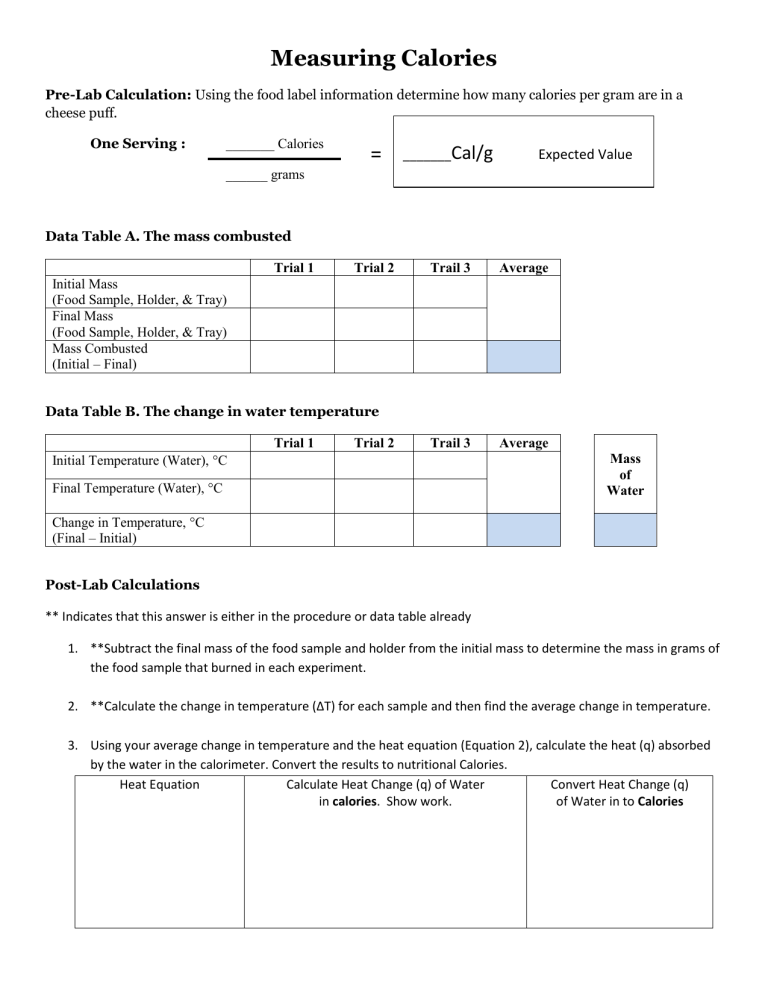
Measuring Calories Pre-Lab Calculation: Using the food label information determine how many calories per gram are in a cheese puff. One Serving : _______ Calories = _______Cal/g Trial 2 Trail 3 Average Trail 3 Average Expected Value ______ grams Data Table A. The mass combusted Trial 1 Initial Mass (Food Sample, Holder, & Tray) Final Mass (Food Sample, Holder, & Tray) Mass Combusted (Initial – Final) Data Table B. The change in water temperature Trial 1 Trial 2 Initial Temperature (Water), C Final Temperature (Water), C Mass of Water Change in Temperature, C (Final – Initial) Post-Lab Calculations ** Indicates that this answer is either in the procedure or data table already 1. **Subtract the final mass of the food sample and holder from the initial mass to determine the mass in grams of the food sample that burned in each experiment. 2. **Calculate the change in temperature (ΔT) for each sample and then find the average change in temperature. 3. Using your average change in temperature and the heat equation (Equation 2), calculate the heat (q) absorbed by the water in the calorimeter. Convert the results to nutritional Calories. Heat Equation Calculate Heat Change (q) of Water Convert Heat Change (q) in calories. Show work. of Water in to Calories 4. Use the average mass combusted (Table A) and your results from Question #3 to calculate the energy content (fuel value) of the food sample in units of Calories per gram (Cal/g). Your Group’s Experimental Value Show your work: 5. Record your group’s result for Question #4 on the board. 6. Calculate the percent error: Percent Error = ( Expected Value – Experimental Value Expected Value ) X 100 Percent Error “Conclusion” Write an organized paragraph that includes the following information: Background/Purpose: State the purpose of the investigation. Describe the combustion reaction. Explain the purpose of combustion reactions in the body and how scientists calculate energy in food. Claim: Does your experimental value for the combustion of the cheese puff match the expected value from the bag? What is causing it to not match? (Remember: “because” is not used in a claim) ________ is causing the percent error to be _____________ Evidence: Describe how far away your experimental value was from the expected value. (% error) Our experimental value did/did not… Our experimental value was… The expected value calculated from the bag information… Therefore we had a ____________ percent error. Reasoning: Explain why you think your percent error was so high. We think our percent error was high because… Solution: Explain the revisions you would make to decrease the percent error in future trials. We believe the percent error would decrease if… because… Claim: Did your solution improve the percent error? (Remember: “because” is not used in a claim) Our solution caused the percent error _____________________ Evidence: Describe how far away your experimental value was from the expected value. (% error) The purpose of this lab was to… Combustion occurs when … creating… Combustion is used for… Scientists calculate energy by… (formula and explain the parts) Our percent error before was… After making corrections to our set up our percent error … Therefore we (increased/decreased) our percent error by __________. Reasoning: Explain why your revisions did or did not decrease the percent error. We believe our percent error (increased/decreased) because…