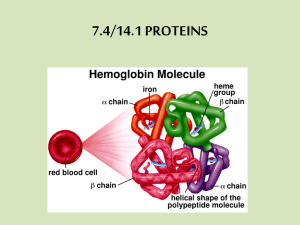
Proteins Proteins are very large molecules (macromolecules) made up of many small monomer units called amino acids joined together by condensation reactions. Amino Acids: All amino acids have the same general structure: there is always an amino group (-NH2) and a carboxyl group (-COOH) covalently bonded to a central alpha carbon atom. The only difference between each amino acid is the nature of the R group or side chain, which varies between amino acids. The structure and composition of the R group therefore defines an amino acid. The R group represents a side chain from the central ‘alpha’ carbon atom, and it can be anything as simple as a single hydrogen atom in glycine to a more complex ring structure. The structure of the R group affects the way the amino acid bonds with others in a protein, depending largely on whether the R group is polar or not. Forming proteins from amino acids: Amino acids join together by a condensation reaction between the carboxyl (COOH) group of one amino acid and the amino (NH2) group of another amino acid, forming a peptide bond between the two amino acids. The result of this joining of two amino acids is a dipeptide. To be more specific, the carbon atom in the hydroxyl group of one amino acid is joined to the nitrogen atom in the amino group of the next by a covalent bond called a peptide bond. This process involves the loss of a water molecule; the carbon atom of the carboxyl group must release an oxygen atom and the nitrogen atom of the amino group must release two hydrogens. These hydrogen and oxygen atoms then combine to form a water molecule. When more and more amino acids are added to a dipeptide, a polypeptide chain is formed. When this polypeptide chain folds or coils into a specific 3D shape, it forms a protein! So essentially, a polypeptide chain is a… And a protein is… Cellular proteins are composed of combinations of 20 different amino acids, each of which can be classified according to the chemical properties of the R group. The particular sequence, or order, in which amino acids are present in a protein determines how it folds into its three dimensional structure. The 3D structure, in turn, determines the protein’s function. Structure in a protein and bonds! There are up to four levels of structure in a protein: primary, secondary, tertiary and quaternary. Each of these play an important role in the overall structure and function of the protein. 1. Primary Structure! A protein’s primary structure is the sequence of amino acids in its polypeptide chain. It is held together by peptide bonds between amino acids. 2. Secondary Structure! The arrangement of polypeptide chains into a regular, repeating 3D structure. Two common arrangements are alpha-helix or Beta-pleated sheets (both types of secondary structures). The shape of the secondary structure is produced and held together by hydrogen bonds that form between nearby amino acids. So, hydrogen bonds create alpha-helix chains and B-pleated sheets. 3. Tertiary Structure! The three-dimensional folding of the secondary structure. The amino acid chain, including many alpha-helices and betapleated sheets, is folded further into complicated shapes. These three-dimensional shapes are held in place by several types of bonds: hydrogen bonds, ionic bonds, disulphide bonds and hydrophobic and hydrophilic interactions between the amino acids. Globular proteins are an example of tertiary structures. 4. Quaternary Structure! The three-dimensional arrangement of more than one tertiary polypeptide! It describes the way these separate polypeptide chains fit together in three dimensions. Held together by hydrogen bonds. Haemoglobin, for example, is made of 4 chains, also known as subunits. More about BONDS – Different bonds hold different structural levels together! 1. Hydrogen Bonds Form between the negatively charged oxygen of the carboxyl groups of one amino acid and the positively charged hydrogen of the amino group of another amino acid. They are weak, but there are lots of them holding the protein together. Hydrogen bonds are easily broken and reformed if pH or temperature conditions change. 2. Disulphide Bonds A strong covalent bond that results from the [oxidation] reaction between two cysteine amino acids. Much stronger than hydrogen bonds, occur much less often. Very important in helping the protein maintain its precise 3D shape. 3. Ionic Bonds Can form between some of the strongly positive and negative amino acid side chains found buried deep in the protein molecules. These links are also known as salt bridges. Form between ionized R groups. Strong bonds, although weaker than disulphide bonds, not as common as other structural bonds. 4. Hydrophobic and hydrophilic interactions: Hydrophobic interactions are between non-polar sections of the protein. When hydrophobic (water-repelling) groups (groups, not molecules or other weird ish) are close together in the protein, they tend to clump together and arrange themselves on the inside of the protein (inside of protein, not polypeptide chain etc.) This means that hydrophilic (water-attracting) groups are more likely to be pushed to the outside, which affects how the protein folds up into its final structure. A protein’s primary structure determines its 3D structure and properties… The amino acid sequence of a protein determines the type of bonds that will form and how the protein will fold up into its 3D structure. E.g. if there are many cysteines, these will form disulphide bonds with each other, so the protein folds up a certain way. In turn, the 3D structure of a protein determines its properties. Protein 3D structures include Globular and Fibrous… 1. Fibrous Proteins! Fibrous proteins have little or no tertiary structure. They are made up of long, parallel polypeptide chains with occasional cross-linkages that form long fibres with high tensile strength and are generally insoluble in water and very tough, which makes them ideally suited to their structural functions within organisms. The chains are held together by lots of bonds (e.g. disulphide and hydrogen bonds) which makes the proteins strong. Because they are strong, fibrous proteins are often found in supportive tissue. For example, collagen is a strong, fibrous protein with 3 alpha chains arranged in a unique triple helix structure held by a very large number of hydrogen bonds. This structure gives it immense strength. It forms supportive tissue in animals. Collagen molecules are also found combined with bone tissue, giving it tensile strength rather like steel rods in reinforced concrete. Fibrous proteins also appear in the structure of connective tissue in tendons and the matrix of bones, as the keratin that makes up hairs, nails, horns and feathers, and in the silk of spiders’ webs and silkworm cocoons. 2. Globular Proteins! Globular Proteins have complex tertiary and quaternary structures (they’re made up of multiple polypeptide chains). They fold into spherical (globular) shapes (so the spherical shape is caused by tightly folded polypeptide chains). The chains are coiled up so that the hydrophilic (water-attracting) parts of the chain are on the outside of the molecule and the hydrophobic (water-repelling) parts of the chain face inwards. This makes the protein soluble, so they’re easily transported in fluids. Examples include transport proteins such as Haemoglobin, which is a globular protein made of 4 polypeptide chains. It carries oxygen around the body in the blood. It’s soluble, so it can be easily transported in the blood. Other examples include Enzymes such as DNA polymerase and ligase and hormones such as oestrogen and insulin. Summary: Denaturing proteins: If the bonds that maintain a protein’s shape are broken, the protein will stop working properly and is denatured. These bonds (that hold the 3D shapes of proteins together) are affected by changes in conditions such as temperature and pH. If these changes cause the bonds to break, the protein loses its 3D shape and is denatured. Fibrous proteins lose their structural strength when denatured, whereas globular proteins become insoluble and inactive. In summary: changes in temp and pH Causes bonds to break 3D strucutre of protein (which is important to their function) is lost (bc it's maintained by bonds) Protein stops working and is denatured Questions… A. Using examples, explain the relationship between structures and function for: 1. Fibrous proteins 2. globular proteins Answer: 1. 2. 3. Fibrous proteins are formed from long, parallel polypeptide chains with some cross-linkages that form fibres. This makes them tough and insoluble in water so they are good structural molecules. Fibrous proteins are formed from long, parallel polypeptide chains with some cross-linkages that form fibres and are held together by lots of bonds which gives them immense strength. This makes them suitable for their structural functions of strength and support. For example, they are found in the supportive tissue of animals and as keratin in nails and hair. Globular proteins have complex structures that form globular shapes. This makes them less soluble in water, so they form colloids which are good for maintaining the structure of the cytoplasm. 7 - Describe the basic structure of an amino acid (structures of specific amino acids are not required) and the formation of polypeptides and proteins (as amino acid monomers linked by peptide bonds in condensation reactions) and explain the significance of a protein’s primary structure in determining its three-dimensional structure and properties (globular and fibrous proteins and types of bonds involved in three-dimensional structure).