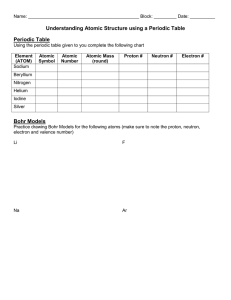
Introduction to Structure of Atom
An atom is present at the most basic level in everything we see around us. In fact,
every living organism is composed of atoms. Every non-living thing around you such as
tables, chairs, water, etc is made up of matter. But the building blocks of matter are atoms.
Therefore, living or non-living, everything is composed of atoms. Let us take a look at the
structure of atom.
Atoms
Atom is a Greek word which means “indivisible.” The Greeks believed that matter can be
broken down into very small invisible particles called atoms. Greek philosophers such as
Democritus and John Dalton put forward the concept of the atom.
Democritus explained the nature of matter. He also proposed that all substances are made up
of matter. He stated atoms are constantly moving, invisible, minuscule particles that are
different in shape, size, temperature and cannot be destroyed.
Atomic Number
Elements are the building blocks for all the matter you see in the world. Now, the question
is how can you distinguish these elements? The answer is “Atomic Number.” Every element
has their unique atomic number that helps to distinguish between two different elements.
Let us study further to know about its significance.
Atomic Number
We know that an atom consists of electrons, protons, and neutrons. Atomic number is one
of the fundamental properties of an atom. Each atom can be characterized by a unique
atomic number. It is represented by the letter “Z.”
The total number of protons present in the nucleus of an atom represents the atomic
number of a particular atom. Every atom of a particular element is composed of the same
number of protons and therefore has the same atomic number. However, atoms of different
elements have unique atomic numbers that vary from one element to the other.
An atom does not have any net charge and is thus electrically neutral. This means that the
number of electrons will be equal to the number of protons present in an atom thereby
making an atom electrically neutral.
“Atomic Number = No. of Protons = No. of Electrons”
For example, each atom of oxygen has 8 protons in their nucleus so the atomic number is 8.
Similarly, the atomic number of carbon is 6 because all atoms of carbon have 6 protons in
their nucleus.
Atomic numbers are whole numbers because it is the total number of protons and protons
are generally units of matter. It ranges from 1 to 118. It starts with hydrogen and ends with
the heaviest known element Oganesson (Og).
Theoretically, the atomic numbers can be increased if more elements are discovered.
However, with the addition of more number of protons and neutrons, the elements become
prone to radioactive decay.
Later in the year 1808, John Dalton proposed the atomic theory and explained the law of
chemical combination. By the end of 18th and the early 20th centuries, many scientists such
as J.J Thomson, Gold stein, Rutherford, Bohr among others developed and proposed several
concepts on “atom.”
Atom is the smallest unit of matter that is composed of a positively charged centre termed
as “nucleus” and the central nucleus is surrounded by negatively charged electrons. Even
though an atom is the smallest unit of matter but it retains all the chemical properties of an
element. For example, a silver spoon is made up of silver atoms with few other constituents.
A silver atom obtains its properties from tiny subatomic particles that it is composed of.
Atoms are further arranged and organized to form larger structures known
as molecules. Atoms, molecules adhere to the general chemistry and physics rule even when
they are part of living breathing human body. Let us study atoms and molecules to further
understand how the atoms react, behave and interact in cells form an essential part of the
living and nonliving world.
Rutherford Atomic Model
Rutherford proposed that an atom is composed of empty space mostly with electrons
orbiting in a set, predictable paths around fixed, positively charged nucleus.
Rutherford’s Atomic Model (Source Credit: Britannica)
History
The concept of atom dates back to 400 BCE when Greek philosopher Democritus first
conceived the idea. However, it was not until 1803 John Dalton proposed again the idea of
the atom. But at that point of time, atoms were considered indivisible. This idea of an atom
as indivisible particles continued until the year 1897 when British Physicist J.J. Thomson
discovered negatively charged particles which were later named electrons.
He proposed a model on the basis of that where he explained electrons were embedded
uniformly in a positively charged matrix. The model was named plum pudding model.
However, J.J. Thomson’s plum pudding model had some limitations. It failed to explain
certain experimental results related to the atomic structure of elements.
A British Physicist “Ernest Rutherford” proposed a model of the atomic structure known as
Rutherford’s Model of Atoms. He conducted an experiment where he bombarded αparticles in a thin sheet of gold. In this experiment, he studied the trajectory of the αparticles after interaction with the thin sheet of gold
Bohr's model of hydrogen
Key points
Bohr's model of hydrogen is based on the non-classical assumption that
electrons travel in specific shells, or orbits, around the nucleus.
Bohr's model calculated the following energies for an electron in the shell, n:
1
𝐸𝑛 = − 2 𝑋13.6𝑒𝑉
𝑛
Bohr explained the hydrogen spectrum in terms of electrons absorbing and
emitting photons to change energy levels, where the photon energy is
ℎ𝑣 = ∇𝐸 = (
1
𝑛𝑖2
−
1
𝑛𝑗2
) 𝑋13. 𝑒𝑉 (Where 𝑛𝑖2 is for low energy and 𝑛𝑗2 is for
high energy).
Bohr's model does not work for systems with more than one electron.
The planetary model of the atom
At the beginning of the 20th century, a new field of study known as quantum
mechanics emerged. One of the founders of this field was Danish physicist
Niels Bohr, who was interested in explaining the discrete line spectrum
observed when light was emitted by different elements. Bohr was also
interested in the structure of the atom, which was a topic of much debate at
the time. Numerous models of the atom had been postulated based on
experimental results including the discovery of the electron by J. J. Thomson
and the discovery of the nucleus by Ernest Rutherford. Bohr supported the
planetary model, in which electrons revolved around a positively charged
nucleus like the rings around Saturn—or alternatively, the planets around the
sun.
Image of Saturn and rings
Many scientists, including Rutherford and Bohr, thought electrons might orbit the nucleus like the rings around
Saturn. Image credit: Image of Saturn by NASA
However, scientists still had many unanswered questions:
Where are the electrons, and what are they doing?
If the electrons are orbiting the nucleus, why don’t they fall into the nucleus as predicted
by classical physics?
How is the internal structure of the atom related to the discrete emission lines produced
by excited elements?
Bohr addressed these questions using a seemingly simple assumption: what if some
aspects of atomic structure, such as electron orbits and energies, could only take on
certain values?
Quantization and photons
By the early 1900s, scientists were aware that some phenomena occurred in a discrete, as
opposed to continuous, manner. Physicists Max Planck and Albert Einstein had recently
theorized that electromagnetic radiation not only behaves like a wave, but also sometimes
like particles called photons. Planck studied the electromagnetic radiation emitted by
heated objects, and he proposed that the emitted electromagnetic radiation was
"quantized" since the energy of light could only have values given by the following
equation: E_{\text{photon}}=nh\nuEphoton=nhν, where nnn is a positive integer, hhh is
Planck’s constant—6.626 \times10^{-34}\,\text {J}\cdot \text s6.626×10−34J⋅s6, point,
626, times, 10, start superscript, minus, 34, end superscript, space, J, dot, s—and \nuν is
the frequency of the light, which has units of \dfrac{1}{\text s}s1start fraction, 1, divided
by, s, end fraction.
As a consequence, the emitted electromagnetic radiation must have energies that are
multiples of h\nuhν. Einstein used Planck's results to explain why a minimum frequency
of light was required to eject electrons from a metal surface in the photoelectric effect.
When something is quantized, it means that only specific values are allowed,
such as when playing a piano. Since each key of a piano is tuned to a specific
note, only a certain set of notes—which correspond to frequencies of sound
waves—can be produced. As long as your piano is properly tuned, you can
play an F or F sharp, but you can't play the note that is halfway between an F
and F sharp.
Atomic line spectra
Atomic line spectra are another example of quantization. When an element or
ion is heated by a flame or excited by electric current, the excited atoms emit
light of a characteristic color. The emitted light can be refracted by a prism,
producing spectra with a distinctive striped appearance due to the emission of
certain wavelengths of light.
Emission spectra of sodium, top, compared to the emission spectrum of the sun, bottom. The dark lines in the
emission spectrum of the sun, which are also called Fraunhofer lines, are from absorption of specific
wavelengths of light by elements in the sun's atmosphere. The side-by-side comparison shows that the pair of
dark lines near the middle of the sun's emission spectrum are probably due to sodium in the sun's atmosphere.
Image credit: From the Biodiversity Heritage Library
For the relatively simple case of the hydrogen atom, the wavelengths of some
emission lines could even be fitted to mathematical equations. The equations
did not explain why the hydrogen atom emitted those particular wavelengths
of light, however. Prior to Bohr's model of the hydrogen atom, scientists were
unclear of the reason behind the quantization of atomic emission spectra.
Bohr's model of the hydrogen atom: quantization of electronic structure
Bohr’s model of the hydrogen atom started from the planetary model, but he added one
assumption regarding the electrons. What if the electronic structure of the atom was
quantized? Bohr suggested that perhaps the electrons could only orbit the nucleus in
specific orbits or shells with a fixed radius. Only shells with a radius given by the
equation below would be allowed, and the electron could not exist in between these
shells. Mathematically, we could write the allowed values of the atomic radius
as r(n)=n^2\cdot r(1)r(n)=n2⋅r(1)r, left parenthesis, n, right parenthesis, equals, n, start
superscript, 2, end superscript, dot, r, left parenthesis, 1, right parenthesis, where nnn is a
positive integer, and r(1)r(1)r, left parenthesis, 1, right parenthesis is the Bohr radius, the
smallest allowed radius for hydrogen.
He found that r(1)r(1)r, left parenthesis, 1, right parenthesis has the value
\text{Bohr radius}=r(1)=0.529 \times 10^{-10}
\,\text{m}Bohr radius=r(1)=0.529×10−10mB, o, h, r, space, r, a, d, i, u, s, equals, r, left
parenthesis, 1, right parenthesis, equals, 0, point, 529, times, 10, start superscript, minus,
10, end superscript, space, m
An atom of lithium shown using the planetary model. The electrons are in circular orbits
around the nucleus. Image credit: planetary atomic model from Wikimedia
Commons, CC-BY-SA 3.0
By keeping the electrons in circular, quantized orbits around the positively-charged
nucleus, Bohr was able to calculate the energy of an electron in the nnnth energy level of
hydrogen: E(n)=-\dfrac{1}{n^2} \cdot 13.6\,\text{eV}E(n)=−n21⋅13.6eVE, left
parenthesis, n, right parenthesis, equals, minus, start fraction, 1, divided by, n, start
superscript, 2, end superscript, end fraction, dot, 13, point, 6, space, e, V, where the
lowest possible energy or ground state energy of a hydrogen electron—E(1)E(1)E, left
parenthesis, 1, right parenthesis—is -13.6\,\text{eV}−13.6eVminus, 13, point, 6, space, e,
V.
Note that the energy is always going to be a negative number, and the ground
state, n=1n=1n, equals, 1, has the most negative value. This is because the energy of an
electron in orbit is relative to the energy of an electron that has been completely separated
from its nucleus, n=\inftyn=∞n, equals, infinity, which is defined to have an energy
of 0\,\text{eV}0eV0, space, e, V. Since an electron in orbit around the nucleus is more
stable than an electron that is infinitely far away from its nucleus, the energy of an
electron in orbit is always negative.
Absorption and emission
The energy level diagram showing transitions for Balmer series, which has
the n=2 energy level as the ground state.
The Balmer series—the spectral lines in the visible region of hydrogen's emission spectrum—corresponds to
electrons relaxing from n=3-6n=3−6n, equals, 3, minus, 6 energy levels to the n=2n=2n, equals, 2 energy level.
Bohr could now precisely describe the processes of absorption and emission
in terms of electronic structure. According to Bohr's model, an electron
would absorb energy in the form of photons to get excited to a higher energy
level as long as the photon's energy was equal to the energy difference
between the initial and final energy levels. After jumping to the higher energy
level—also called the excited state—the excited electron would be in a less
stable position, so it would quickly emit a photon to relax back to a lower,
more stable energy level.
The energy levels and transitions between them can be illustrated using
an energy level diagram, such as the example above showing electrons
relaxing back to the n=2n=2n, equals, 2 level of hydrogen. The energy of the
emitted photon is equal to the difference in energy between the two energy
levels for a particular transition. The energy difference between energy
levels n_{high}nhighn, start subscript, h, i, g, h, end subscript and n_{low}nlow
n, start subscript, l, o, w, end subscript can be calculated using the equation
for E(n)E(n)E, left parenthesis, n, right parenthesis from the previous section:
\begin{aligned} \Delta E &= E(n_{high})-E(n_{low}) \\ \\ &=\left(
-\dfrac{1}{{n_{high}}^2} \cdot 13.6\,\text{eV} \right)-\left(\dfrac{1}{{n_{low}}^2} \cdot 13.6\,\text{eV}\right) \\ \\ &=
\left(\dfrac{1}{{n_{low}}^2}-\dfrac{1}{{n_{high}}^2}\right) \cdot
13.6\,\text{eV} \end{aligned}ΔE=E(nhigh)−E(nlow)=(−nhigh21
⋅13.6eV)−(−nlow21⋅13.6eV)=(nlow21−nhigh21)⋅13.6eV
Since we also know the relationship between the energy of a photon and its
frequency from Planck's equation, we can solve for the frequency of the
emitted photon:
\begin{aligned} h\nu &=\Delta E = \left(\dfrac{1}{{n_{low}}^2}\dfrac{1}{{n_{high}}^2}\right) \cdot 13.6\,\text{eV}
~~~~~~~~~~~~\text{Set photon energy equal to energy
difference}\\ \\ \nu &= \left(\dfrac{1}{{n_{low}}^2}\dfrac{1}{{n_{high}}^2}\right) \cdot
\dfrac{13.6\,\text{eV}}{h}~~~~~~~~~~~~~~~~~~~~~~\text{Solve
for frequency}\end{aligned}hνν=ΔE=(nlow21−nhigh21
)⋅13.6eV
Set photon energy equal to energy difference=(n
low21−nhigh21)⋅h13.6eV
Solve for frequency
We can also find the equation for the wavelength of the emitted
electromagnetic radiation using the relationship between the speed of
light \text ccc, frequency \nuν, and wavelength \lambdaλlambda:
\begin{aligned}\text c &=\lambda \nu
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
~~~~~~~~~~~~~~\text{Rearrange to solve for }\nu . \\
\dfrac{\text c}{\lambda}&=\nu=\left(\dfrac{1}{{n_{low}}^2}\dfrac{1}{{n_{high}}^2}\right) \cdot
\dfrac{13.6\,\text{eV}}{h}~~~~~~~~~~~~~~\text{Divide both
sides by c to solve for }\dfrac{1}{\lambda}.\\ \\
\dfrac{1}{\lambda} &=\left(\dfrac{1}{{n_{low}}^2}\dfrac{1}{{n_{high}}^2}\right) \cdot
\dfrac{13.6\,\text{eV}}{h\text c} \end{aligned}cλcλ1
=λν
Rearrange to solve for ν
.=ν=(nlow21−nhigh21)⋅h13.6eV
Divide both sides by c to solve for λ1.=(nlow21−nhigh21
)⋅hc13.6eV
[What about the Rydberg constant?]
RR
R=\dfrac{13.6\,\text{eV}}{h\text c}=1.1\times 10^7\,\dfrac{1}{m}R, equals,
start fraction, 13, point, 6, space, e, V, divided by, h, c, end fraction, equals,
1, point, 1, times, 10, start superscript, 7, end superscript, space, start
fraction, 1, divided by, m, end fraction
RR
\dfrac{1}{\lambda}=\left(\dfrac{1}{{n_{low}}^2}\dfrac{1}{{n_{high}}^2}\right) \cdot Rstart fraction, 1, divided by, lambda,
end fraction, equals, left parenthesis, start fraction, 1, divided by, n, start
subscript, l, o, w, end subscript, start superscript, 2, end superscript, end
fraction, minus, start fraction, 1, divided by, n, start subscript, h, i, g, h, end
subscript, start superscript, 2, end superscript, end fraction, right parenthesis,
dot, R
RRccE(1)E, left parenthesis, 1, right parenthesishh
Thus, we can see that the frequency—and wavelength—of the emitted photon
depends on the energies of the initial and final shells of an electron in
hydrogen.
What have we learned since Bohr proposed his
model of hydrogen?
The Bohr model worked beautifully for explaining the hydrogen atom and
other single electron systems such as \text{He}^+He+H, e, start superscript,
plus, end superscript. Unfortunately, it did not do as well when applied to the
spectra of more complex atoms. Furthermore, the Bohr model had no way of
explaining why some lines are more intense than others or why some spectral
lines split into multiple lines in the presence of a magnetic field—the Zeeman
effect.
In the following decades, work by scientists such as Erwin Schrödinger
showed that electrons can be thought of as behaving like waves and behaving
as particles. This means that it is not possible to know both a given electron’s
position in space and its velocity at the same time, a concept that is more
precisely stated in Heisenberg's uncertainty principle. The uncertainty
principle contradicts Bohr’s idea of electrons existing in specific orbits with a
known velocity and radius. Instead, we can only calculate probabilities of
finding electrons in a particular region of space around the nucleus.
The modern quantum mechanical model may sound like a huge leap from the
Bohr model, but the key idea is the same: classical physics is not sufficient to
explain all phenomena on an atomic level. Bohr was the first to recognize this
by incorporating the idea of quantization into the electronic structure of the
hydrogen atom, and he was able to thereby explain the emission spectra of
hydrogen as well as other one-electron systems.
Copyright © Michael Richmond. This work is licensed under a Creative
Commons License.
Heisenberg's Microscope
This lecture describes a hypothetical microscope that Werner Heisenberg used to
explain his Uncertainty Principle. A good place to learn more about Heisenberg and
his ideas is the AIP History of Heisenberg.
Copyright © Michael Richmond. This work is licensed under a Creative
Commons License.