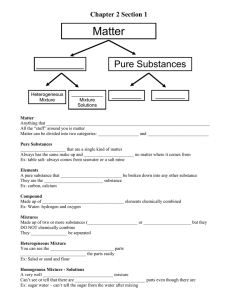
Chapter 3 – Matter and Energy Denotes – not necessary to copy Matter States of Matter: solid – liquid – gas increasing energy >>>> 50mL of ice has less energy than 50mL of water. 50mL of water has less energy than 50mL of steam. The energy of the particles in the 50mL of H2O increases as it goes from solid-liquid-gas. There are two questions that can be answered to determine the state of matter. 1. Does the matter take the shape of its container? 2. Does the volume of the matter remain constant? If the answer to both questions is yes; the state of the matter is a gas. If the answer to both questions is no; the state of the matter is a solid. If the sample of matter takes the shape of its container but has constant volume; the state of the matter is a liquid. A liquid shares one property of each other the other states of matter. Probably the most important real world lesson involving states of matter is that a liquid CANNOT be compressed. When a liquid gets in a place (engine cylinder) where a gas is to be compressed, something had to give and it usually causes severe engine damage. This could happen if a car runs through deep water. Change of State Chart Change from: Solid Liquid Gas Solid to a Melting Sublimation Liquid to a Freezing Vaporization Gas to a Deposition Condensation Note: Vaporization includes evaporation (when particles at the surface gain enough energy to transition from liquid to gas) and boiling (particles throughout the substance gain enough energy to transition from liquid to gas) Physical and Chemical Properties and Changes Physical property – a property based on the state of a substance. All of the changes listed in the chart above are physical changes. Also any change that does not involve a substance becoming a different substance is a physical change. Cutting a paper into pieces is a physical change. Grating cheese is a physical change. Chemical property – a property based on the reactivity of a substance. A chemical change is called a chemical reaction. The requirement for a change to be a chemical change is the formation of at least one new substance. The nail rusted away to nothing. This indicates a chemical reaction; Fe + O2 Fe2O3 Note: Burn words typically indicate a chemical change has occurred. Scorched, burnt, bake, roast, sear, toast, etc. Classification of Matter Pure Substance – the word substance implies pure substance in chemistry. Element – a pure substance that cannot be broken down into simpler substances by chemical means Compound – a pure substance that can be broken down into simpler substances by chemical means Fe + O2 Fe2O3 (a synthesis reaction is when two pure substances are chemically combined to form a compound) H2O H2 + O2 (a decomposition reaction is when a compound is separated into smaller pure substances) Mixture – matter that has variable composition KoolAid is a mixture it can be sweeter, more watery, stronger tasting, etc. Pure Substance – matter that always has the same composition; pure water is always H2O. Water is typically a mixture, especially tap water. Tap water contains many chemicals. Some added to make the water healthier and some trace elements that are too expensive to remove. A mixture can be separated into two or more pure substances by physical means. Homogeneous mixture – called a solution, does not vary in composition from one region to another. Pepsi is an example of a homogeneous mixture. Every drink you get from the bottle is the same. One isn’t sweeter, or more cola tasting. Heterogeneous mixture – contains regions that have different properties from one another. Examples range from trail mix to Italian salad dressing. Whenever you can isolate different samples from the mixture you are dealing with a heterogeneous mixture. Separation of Mixtures Distillation is used to separate the pure substances from a homogeneous mixture. Distillation takes advantage of the physical property, boiling point. Filtration is used to separate the components of a heterogeneous mixture. Filtration takes advantage of particle size. Energy, Temperature, and Heat Energy is the capacity to do work. Heat is the flow of energy due to a temperature difference. Exothermic – energy exits the system to the surroundings (the surroundings get hotter) Endothermic – energy enters the system from the surroundings (the surroundings get cooler) Tfinal = T(hot) + T(cold) 2 Calculating Energy Changes 𝑸 = 𝒎𝒄∆𝑻 𝑸 = energy 𝒎 = mass 𝒄 = the specific heat capacity for the sample of matter ∆𝑻 = change in temperature = Tfinal – Tinitial Here are the units associated with the variables above: energy joules (J) mass grams the specific heat capacity J/g-°C change in temperature °C A. The specific heat capacity for water is 4.184 J/g°C. How much energy would be required to raise the temperature of 255.8 g of water from 20.0°C to 77.6°C? 𝑸 = 𝒎𝒄∆𝑻 𝑸 = 255.8 g (4.184 J/g-°C) (77.6°C – 20.0°C) 𝑸 = 255.8 g (4.184 J/g-°C) (57.6°C) 𝑸 = 61600 J The specific heat capacity is the amount of energy required to raise the temperature of one gram of a substance by one degree Celsius. 4.184 joules = 1 calorie (a calorie is bigger than a joule) I eat about 3,000,000 calories. That is 3000 kilocalories. Nutritional calories are really kilocalories. On a food label they will always be listed as a capital C as in 100 Cal. That really means 100000 calories. A can of Pepsi has 100000 calories!!! B. A 43.75 g sample of aluminum is heated by adding 5.58 X 103 J. The initial temperature of the aluminum was 22.8°C what was the final temperature of the aluminum?(See page 70 for 𝒄Al) EOCQ’s p.75 7,9,10,11,16, 17, 19, 27-31,34,35,38,39,41,43,44,55,59,61 Lab Experiments 1. Filtration/Distillation 2. Specific Heat Virtual Lab