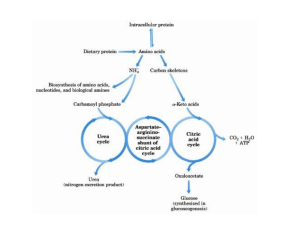
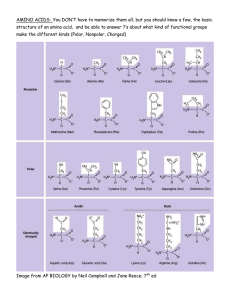
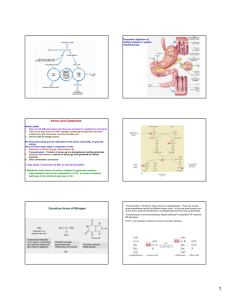
Lecture Notes; Urea Cycle Biochemistry 2, 2016 FV All tissues have some capability for synthesis of the non-essential amino acids, amino acid by remodeling, and the conversion of non-amino acid carbon skeletons into amino acids and other derivatives that contain nitrogen. However, the liver is the major site of nitrogen metabolism in the body. In times of dietary surplus, the potentially toxic nitrogen of amino acids is eliminated via transamination, deamination, and urea formation; the carbon skeletons are generally conserved as carbohydrate, via gluconeogenesis, or as fatty acid via fatty acid synthesis pathways. In this respect amino acids fall into three categories: glucogenic, ketogenic, or glucogenic and ketogenic. (a)The parietal cells and chief cells of the gastric glands secrete their products in response to the hormone gastrin. Pepsin begins the process of protein degradation in the stomach. (b) The cytoplasm of exocrine cells is completely filled with rough endoplasmic reticulum, the site of synthesis of the zymogens of many digestive enzymes. The zymogens are concentrated in membrane-enclosed transport particles called zymogen granules. When an exocrine cell is stimulated, its plasma membrane fuses with the zymogen granule membrane and zymogens are released into the lumen of the collecting duct by exocytosis. The collecting ducts ultimately lead to the pancreatic duct and thence to the small intestine. (c) Amino acids are absorbed through the epithelial cell layer (intestinal mucosa) of the villi and enter the capillaries. Recall that the products of lipid hydrolysis in the small intestine enter the lymphatic system after their absorption by the intestinal mucosa. The glucose-alanine cycle is used primarily as a mechanism for skeletal muscle to eliminate nitrogen while replenishing its energy supply. Glucose oxidation produces pyruvate which can undergo transamination to alanine. This reaction is catalyzed by alanine transaminase, ALT (ALT used to be called serum glutamatepyruvate transaminase, SGPT, this is different from the transaminase SGOT). Additionally, during periods of fasting, skeletal muscle protein is degraded for the energy value of the amino acid carbons and alanine is a major amino acid in protein. The alanine then enters the blood stream and is transported to the liver. Within the liver alanine is converted back to pyruvate which is then a source of carbon atoms for gluconeogenesis. The newly formed glucose can then enter the blood for delivery back to the muscle. The amino group transported from the muscle to the liver in the form of alanine is converted to urea in the urea cycle and excreted. mTOR kinase is a master growth regulator that can be stimulated by multiple signals, including amino acids and the lysosomal small GTPase Rheb. Recent studies have proposed an important role for the V-ATPase in the sensing of amino acids in the lysosomal lumen. It shows here that the V-ATPase is required for Rheb-dependent epithelial growth. Further uncover a positive feedback loop for the control of apical protein uptake that depends on V-ATPase/mTOR signaling. This feedback loop includes Rheb-dependent transcriptional regulation of the multiligand receptor Megalin, which itself is required for Rheb-induced endocytosis. In addition, we provide evidence that long-term mTOR inhibition with rapamycin in mice causes reduction of Megalin levels and proteinuria in the proximal tubular epithelium of the kidney. Mechanism of Transaminase Reaction, Schiff Base Rxn, forming transiently pyridoamine PO4. GS catalyzes the ATP-dependent condensation of glutamate with ammonia to yield glutamine. The hydrolysis of ATP drives the first step of a two-part, concerted mechanism. ATP phosphorylates glutamate to form ADP and an acyl-phosphate intermediate, γ-glutamyl phosphate, which reacts with ammonia, forming glutamine and inorganic phosphate. ADP and Pi do not dissociate until ammonia binds and glutamine is released. GS is present predominantly in the brain, kidneys, and liver. Glutamine Synthetase can be composed of 8, 10, or 12 identical subunits separated into two face-to-face rings. Each active site creates a ‘bifunnel’ which is the site of three distinct substrate binding sites: nucleotide, ammonium ion, and amino acid. ATP binds to the top of the bifunnel that opens to the external surface of GS. Glutamate binds at the bottom of the active site. The middle of the bifunnel contains two sites in which divalent cations bind (Mn+2 or Mg+2). One cation binding site is involved in phosphoryl transfer of ATP to glutamate, while the second stabilizes active GS and helps with the binding of glutamate. LAAOs are flavoenzymes which function to catalyze the stereospecific oxidative deamination of an L-amino acid. sv-LAAOs are often found as functional dimers, with each dimer having three domains. The three domains are the substratebinding site, FAD-binding site, and a helical domain. This enzyme participates in 8 metabolic pathways: alanine and aspartate metabolism, methionine metabolism, valine, leucine and isoleucine degradation, tyrosine metabolism, phenylalanine metabolism, tryptophan metabolism, phenylalanine, tyrosine and tryptophan biosynthesis. GABA transaminase enzyme catalyzes the conversion of 4-aminobutanoic acid (GABA) and 2-oxoglutarate (α-ketoglutarate) into succinic semialdehyde and glutamate. Succinic semialdehyde is then oxidized into succinic acid by succinic semialdehyde dehydrogenase and as such enters the TCA cycle as a usable source of energy. Short-term regulation of the cycle occurs principally at CPS-I, which is inactive in the absence of its obligate activator N-acetylglutamate. The steady-state concentration of Nacetylglutamate is set by the concentration of its components acetyl-CoA and glutamate and by arginine, which is a positive allosteric effector of N-acetylglutamate synthase(NAGS). The ammonia produced in the latter two reactions can be excreted as NH4+ in the urine, where it helps maintain urine pH in the normal range of pH 4 to pH 8. The extensive production of ammonia by peripheral tissue or hepatic glutamate dehydrogenase is not feasible because of the highly toxic effects of circulating ammonia. Normal serum ammonium concentrations are in the range of 20–40μM, and an increase in circulating ammonia to about 400μM causes alkalosis and neurotoxicity. Schematic view and structure of the cKGA-L-glutamine complex. (A) Human KGA domains and signature motifs. (B) Structure of the of Hu Kidney Glutaminase (cKGA) and bound substrate (Lglutamine) is shown as a cyan stick. (C) Electron density map for L-glutamine, that makes hydrogen bonds with active site residues are shown. Glutaminase is expressed and active in periportal hepatocytes, where it generates NH3 (ammonia) for urea synthesis, as does glutamate dehydrogenase.[2] Glutaminase is also expressed in the epithelial cells of the renal tubules, where the produced ammonia is excreted as ammonium ions. This excretion of ammonium ions is an important mechanism of renal acid-base regulation. During chronic acidosis, glutaminase is induced in the kidney, which leads to an increase in the amount of ammonium ions excreted. Glutaminase can also be found in the intestines, whereby hepatic portal ammonia can reach as high as 0.26 mM (compared to an arterial blood ammonia of 0.02 mM). ADP is the strongest adenine nucleotide activator of glutaminase. Studies have also suggested ADP lowered the K(m) for glutamine and increased the V(max). They found that these effects were increased even more when ATP was present. Phosphate-activated mitochondrial glutaminase (GLS2) is suggested to be linked with elevated metabolism, decreased intracellular reactive oxygen species (ROS) levels, and overall decreased DNA oxidation in both normal and stressed cells. It is suggested that GLS2’s control of ROS levels facilitates “the ability of p53 to protect cells from accumulation of genomic damage and allows cells to survive after mild and repairable genotoxic stress.” CPS is technically not part of the Urea cycle. It forms carbamylPO4 which is one of the substrates of the Ornithine transcarbamylase. Eurkaryotes have two forms of the enzyme, CPSI is found in the mitochondria matrix, CPSII is cytosolic and part of pyrimidine synthesis. CPSI uses NH3 while CPSII used glutamine as the Nitrogen donor. The CPSI rxn is non-reversible and is the allosteric and rate limiting step of the Urea Cycle. E.coli has only one CPS which is homologous to both enzymes found in eukaryotes. The enzyme exhibits substrate tunneling. The three sites of the rxn are found along a 96A tunnel in the elongate protein. X-Ray structure of E. coli carbamoyl phosphate synthetase (CPS). The active site holds L-arginine in place via hydrogen bonding between the guanidinium group with Glu227. This bonding orients L-arginine for nucleophilic attack by the metalassociated hydroxide ion at the guanidinium group. This results in a tetrahedral intermediate. The manganese ions act to stabilize both the hydroxyl group in the tetrahedral intermediate, as well as the developing sp3 lone electron pair on the NH2 group as the tetrahedral intermediate is formed. Arginase's active site is extraordinarily . specific. The pyridoxal phosphate cofactor binds lysine 69 at the C-terminus end of the barrel domain. The active site is at the interface of the two domains, in a cavity formed by loops from both monomers The glucose-alanine cycle is used primarily as a mechanism for skeletal muscle to eliminate nitrogen while replenishing its energy supply. Glucose oxidation produces pyruvate which can undergo transamination to alanine. This reaction is catalyzed by alanine transaminase, ALT (ALT used to be called serum glutamatepyruvate transaminase, SGPT, this is different from the transaminase SGOT). Additionally, during periods of fasting, skeletal muscle protein is degraded for the energy value of the amino acid carbons and alanine is a major amino acid in protein. The alanine then enters the blood stream and is transported to the liver. Within the liver alanine is converted back to pyruvate which is then a source of carbon atoms for gluconeogenesis. The newly formed glucose can then enter the blood for delivery back to the muscle. The amino group transported from the muscle to the liver in the form of alanine is converted to urea in the urea cycle and excreted. Nitrogen-acquiring reactions in the synthesis of urea. The urea nitrogens are acquired in two reactions, each requiring ATP. (a) In the reaction catalyzed by carbamoyl phosphate synthetase I, the first nitrogen enters from ammonia. The terminal phosphate groups of two molecules of ATP are used to form one molecule of carbamoyl phosphate. In other words, this reaction has two activation steps (1 and 3). (b) In the reaction catalyzed by argininosuccinate synthetase, the second nitrogen enters from aspartate. Activation of the ureido oxygen of citrulline in step 1 sets up the addition of aspartate in step 2.