Ionic & Molecular Compounds, Chemical Reactions Study Guide
advertisement

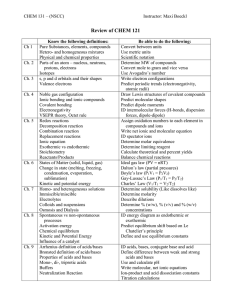
Ionic and Molecular Compounds ● Understand the difference between the two – be able to explain how they work and what type of bond they have o Ionic ▪ Ionic Bond ▪ Bond between a nonmetal and a metal or a polyatomic and a metal ▪ bonded by electromagnetic energy ▪ metals lose electrons and become positive ions ▪ nonmetals gain electrons and become negative ions ▪ Chemical Formulas o Molecular ▪ Covalent bond ▪ bond between two nonmetals ▪ bonded by sharing electrons ▪ electrons exist in both atoms’ shells to give them full shells ▪ do not become ions ● Know their properties o Ionic ▪ High melting and boiling points ▪ Ionic bonds are very strong - a lot of energy is needed to break them ▪ Conductive in liquid state ▪ Make lattice shape o Covalent ▪ Low melting points ▪ Non conductive ( not ionized) ▪ Usually insoluble ● Be able to state their Chemical Name and Formula o ionic ▪ criss-cross valence to get number of atoms for each element ▪ name multivalent metals with roman numerals o Chemical Formulas ▪ ▪ name by taking how many of each atom and using the corresponding prefix no criss cross Law of Conservation of Mass ● Understand what this is and be able to explain it o The law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as system's mass cannot change, so quantity cannot be added or removed. Hence, the quantity of mass is conserved over time. ● Balance Chemical Equations o make sure there are the same amount of each element on both sides of the equation o use coefficients in front of the compounds to multiply how many atoms there are Chemical Equations/Reactions ● Understand/know the evidence that a chemical reaction has taken place o Change in energy ▪ temperature ▪ light ▪ electricity o change in colour o gas is given off ▪ bubbles ▪ smell o solid (precipitate) formed ● Be able to go from a sentence to word equation, to a skeleton equation, to a balanced chemical equation ● Remember the diatomic elements o I Have No Bright Or Clever Friends o Diatomic 7 ▪ forms a 7 on the periodic table (also hydrogen) o Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine, Bromine, Iodine ● Understand the difference between an endothermic and exothermic reaction and be able to state what type of reaction it is o endothermic ▪ requires more energy to react than it produces ▪ gets colder ▪ usually decomposition reaction (breaking bonds) o exothermic ▪ releases energy ▪ forming bonds releases energy ▪ could be synthesis reaction ▪ could be combustion ● Know the different Types of Reactions – be able to explain them, recognize them and develop them o Synthesis ▪ A+B→ AB o Decomposition ▪ AB→ A+B o Combustion ▪ CxHy+ O2→ CO2 + H2O o Single Displacement ▪ AB + C → A + CB o Double Replacement ▪ AB + CD → CB + AD ▪ Solubility Rules ● Most Double Displacement/ Replacement reactions involve aqueous solutions of ionic compounds ● For a reaction to occur a precipitate must form ( insoluble solid) ● To predict which compound will be soluble and which forms the precipitate we use these rules: o All compounds with nitrate (NO3-) are soluble o all compounds with ammonium (NH4+) are soluble o All compounds with group 1 metals ( alkali ) are soluble o Most other compounds are insoluble o Neutralization ▪ Acid + Base → Water + Salt Acid and Bases ● Be able to identify them o Acids ▪ Start with “H” or end with “COOH”(organic acid) ▪ are in an aqueous state (aq) ▪ any ionic compound with an H+ ion that is dissolved in water becomes an acid o Bases ▪ have hydroxide in them (OH) ▪ when dissolved in water produce OH- ions ● Know their properties o acids ▪ have pH below 7 ▪ sour ▪ corrosive ▪ aqueous solutions ▪ water soluble ▪ good conductor ▪ made when an acid oxide reacts with water (ClO + H20 → HClO2) ● acid oxides are any non metal mixed with oxygen o bases ▪ have pH above 7 ▪ bitter ▪ slippery ▪ water soluble ▪ good conductor ▪ made when a basic oxide reacts with water ( NaO + H20 → NaOH) ● basic oxides are any metal mixed with oxygen ● Understand what Indicators and the pH scale are – be able to answer related questions o Indicators ▪ Litmus paper ● Blue o turns red when in contact with acids ● Red o turns blue in contact with bases ▪ Phenolphthalein solution ● turns pink in contact with bases ▪ bromothymol blue ● turns blue in contact with bases ● turns yellow in contact with acids ● turns green in contact with neutral solutions o pH scale ▪ ▪ ▪ goes from 0-14 lower the number the more acidic each number increases in a power of ten (eg. pH of 5 is 10x more acidic than pH of 6) ▪ pH stands for “Power of Hydrogen” ● Be able to state their Chemical Name and Formula o Acids ▪ If the name of the ionic compound is “Hydrogen ide” ● becomes Hydro ic Acid ▪ If the name of the ionic compound is “Hydrogen ate” ● becomes ic Acid ▪ If the name of the ionic compound is “Hydrogen ide” ● becomes ous Acid ● Understand how a neutralization reaction takes place and be able to explain its importance o Neutralization takes place when an acid and a base mix o the H+ ion mixes with the OH- ion to make water, the other two atoms mix to form a salt o Salt ▪ Salts are ionic compounds formed when: ● acids and bases react ● OR when oxides or carbonates react with acids ● OR when metals react with acids. ▪ Table salt, (NaCl) is only one kind of salt: ● it is found in sea water, salt lakes or rock deposits. ▪ Salts are found in many things: ● In batteries, explosives and fertilizers ● In multivitamins ● in many living cells o Importance of Neutralization ▪ Antacids ● The pits in the lining of your stomach secrete hydrochloric acid. ● The acid is used in the breakdown and digestion of food. ● If someone suffers from an excess production of acid, it’s called heartburn. ▪ If an acid or base is released and we need to get rid of it we can neutralize it with a base or acid.