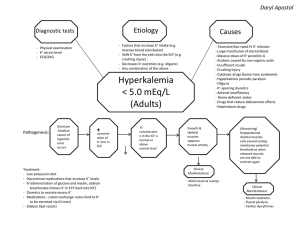
Keseimbangan Air dan Natrium Figure 27-1a The Composition of the Human Body SOLID COMPONENTS (31.5 kg; 69.3 lbs) Kg Proteins Lipids Minerals Carbohydrates Miscellaneous The body composition (by weight, averaged for both sexes) and major body fluid compartments of a 70-kg individual. Figure 27-1a The Composition of the Human Body WATER (38.5 kg; 84.7 lbs) Other Plasma Liters Interstitial fluid Intracellular fluid Extracellular fluid The body composition (by weight, averaged for both sexes) and major body fluid compartments of a 70-kg individual. Figure 27-1b The Composition of the Human Body WATER 60% ICF ECF Intracellular fluid 33% Interstitial fluid 21.5% Plasma 4.5% Solids 40% (organic and inorganic materials) Other body fluids (1%) SOLIDS 40% Adult males A comparison of the body compositions of adult males and females, ages 18–40 years. Figure 27-1b The Composition of the Human Body WATER 50% ECF ICF Intracellular fluid 27% Interstitial fluid 18% Plasma 4.5% Solids 50% (organic and inorganic materials) Other body fluids (1%) SOLIDS 50% Adult females A comparison of the body compositions of adult males and females, ages 18–40 years. Figure 27-2 Cations and Anions in Body Fluids CATIONS ICF ECF KEY Na Cations Na Milliequivalents per liter (mEq/L) K Ca2 Mg2 K Na K Ca2 Plasma Na K Interstitial fluid Mg2 Intracellular fluid Figure 27-2 Cations and Anions in Body Fluids ANIONS ECF ICF KEY Anions HCO3 Cl HCO3 Cl HPO42 HCO3 SO42 HCO3 Organic acid HPO42 Proteins Cl Cl HPO42 Org. acid Proteins Plasma SO42 Proteins HPO42 SO42 Interstitial fluid Intracellular fluid Figure 27-3 Fluid Gains and Losses Water absorbed across digestive epithelium (2000 mL) Water vapor lost in respiration and evaporation from moist surfaces (1150 mL) ICF Metabolic water (300 mL) ECF Water lost in feces (150 mL) Water secreted by sweat glands (variable) Plasma membranes Water lost in urine (1000 mL) Table 27-1 Water Balance Figure 27-4 Fluid Shifts between the ICF and ECF Intracellular fluid (ICF) Extracellular fluid (ECF) The ECF and ICF are in balance, with the two solutions isotonic. Decreased ECF volume Water loss from ECF reduces volume and makes this solution hypertonic with respect to the ICF. Decreased ICF volume Increased ECF volume An osmotic water shift from the ICF into the ECF restores osmotic equilibrium but reduces the ICF volume. Figure 27-5 The Homeostatic Regulation of Normal Sodium Ion Concentrations in Body Fluids ADH Secretion Increases Recall of Fluids The secretion of ADH restricts water loss and stimulates thirst, promoting additional water consumption. Because the ECF osmolarity increases, water shifts out of the ICF, increasing ECF volume and lowering Na concentrations. Osmoreceptors in hypothalamus stimulated HOMEOSTASIS RESTORED HOMEOSTASIS DISTURBED Decreased Na levels in ECF Na Increased levels in ECF HOMEOSTASIS Normal Na concentration in ECF Start Figure 27-5 The Homeostatic Regulation of Normal Sodium Ion Concentrations in Body Fluids HOMEOSTASIS HOMEOSTASIS DISTURBED Normal Na concentration in ECF Start HOMEOSTASIS RESTORED Decreased Na levels in ECF Osmoreceptors in hypothalamus inhibited Increased Na levels in ECF Water loss reduces ECF volume, concentrates ions ADH Secretion Decreases As soon as the osmotic concentration of the ECF drops by 2 percent or more, ADH secretion decreases, so thirst is suppressed and water losses at the kidneys increase. Figure 27-6 The Integration of Fluid Volume Regulation and Sodium Ion Concentrations in Body Fluids Responses to Natriuretic Peptides Increased Na loss in urine Rising blood pressure and volume Increased water loss in urine Natriuretic peptides released by cardiac muscle cells Reduced thirst Inhibition of ADH, aldosterone, epinephrine, and norepinephrine release Combined Effects Reduced blood volume Reduced blood pressure Increased blood volume and atrial distension HOMEOSTASIS DISTURBED HOMEOSTASIS RESTORED Rising ECF volume by fluid gain or fluid and Na gain Falling ECF volume HOMEOSTASIS Start Normal ECF volume Figure 27-6 The Integration of Fluid Volume Regulation and Sodium Ion Concentrations in Body Fluids HOMEOSTASIS Start Normal ECF volume HOMEOSTASIS DISTURBED Falling ECF volume by fluid loss or fluid and Na loss Decreased blood volume and blood pressure Falling blood pressure and volume HOMEOSTASIS RESTORED Rising ECF volume Endocrine Responses Combined Effects Increased renin secretion and angiotensin II activation Increased urinary Na retention Decreased urinary water loss Increased thirst Increased water intake Increased aldosterone release Increased ADH release Table 27-2 Electrolyte Balance for Average Adult Scientific Knowledge Base : Location and Movement of Water and Electrolytes Intracellular Fluid (ICF) Extracellular Fluid (ECF) = Fluid outside of cells = Fluids within cells ~2/3 of total body water ~1/3 of total body water Three divisions: – Interstitial – Intravascular – Transcellular Distribution and Composition of Body Fluids • Intracellular • Solute • Extracellular • Vascular, interstitial fluid Movement of Body Fluids • Movement of body fluid across cell and capillary membranes accomplished by: • • • • Osmosis Diffusion Filtration Active transport Movement of Body Fluids, continued • Osmosis Movement of water across cell membranes from less concentrated solution to more concentrated solution Solutes: crystalloids, colloids Solvent: component of solution that can dissolve a solute Check out this link to Khan academy on Osmosis/Diffusion Movement of Body Fluids, continued • Osmosis • Osmolality: concentration of solutes • Greatest determinants of osmolality in ECF: sodium, glucose, urea • Greatest determinants of osmolality in ICF: potassium, glucose, urea Movement of Body Fluids, continued • Osmosis • Tonicity: osmolality of solution • Isotonic solution: same osmolality as body fluids • Hypertonic solution: higher osmolality than body fluids • Hypotonic solution: lower osmolality than body fluids Isotonic, Hypotonic, and Hypertonic Solutions Movement of Body Fluids, continued • Osmosis • Osmotic pressure: power of solution to draw water across membrane • Colloid osmotic pressure (oncotic pressure): plasma proteins pull water from interstitial space into vascular compartment • Important in maintaining vascular volume Movement of Body Fluids, continued • Diffusion • Intermingling of molecules • Rate of diffusion varies by: • Size of molecules • Concentration of solution • Temperature of solution Movement of Body Fluids, continued • Filtration • Movement of fluid and solutes together across a membrane from one compartment to another • From area of higher pressure to lower • Hydrostatic pressure: pressure a fluid exerts on walls in closed system Movement of Body Fluids, continued • Active transport • Substances move across membranes • From less concentrated solution to more concentrated one • Metabolic energy is expended • Maintains higher sodium levels in ECF, higher potassium concentrations in ICF • Sodium-potassium pump PENGARUH OSMOLARITAS PADA SEL faal_cairan-asam-basa/ikun/2006 29 Komposisi Ion pd Cairan Tubuh faal_cairan-asam-basa/ikun/2006 30 faal_cairan-asam-basa/ikun/2006 31 Peranan ginjal faal_cairan-asam-basa/ikun/2006 32 Filtrasi, Reabsorpsi, Sekresi & Ekskresi di Nefron faal_cairan-asam-basa/ikun/2006 33 Peranan Renin-Angiotensin-Aldosteron faal_cairan-asam-basa/ikun/2006 34 Respons thd Asupan Garam faal_cairan-asam-basa/ikun/2006 35