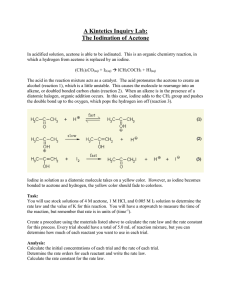
EXPERIMENT 1: REACTION KINETICS OF BROMINATION OF ACETONE OBJECTIVE 1. To determine the rate, order and activation energy of the bromination of acetone. EXPERIMENTAL PROCEDURE A. Determination of reaction rate at room temperature 1. 10 mL of 4M acetone is filled into a boiling tube labeled as A,followed by 10 mL of 1.0M HCl and 20 mL of distilled water. 2. 10 mL of 0.02M bromine solution is measured using graduated measuring cylinder and poured into another boiling tube (B). 3. Both boiling tubes is immersed in water bath at room temperature about 5 minutes until the solution have reached the temperature of water bath. 4. The two solution is mixed together as the stopwatch started to record the exact time of mixing. 5. The time,t is recorded when the reaction is complete, that is when the solution becomes colourless. A test tube filled with distilled water is used for colour comparison. 6. Step 1 to 5 is repeated twice to ensure that reproducible results are obtained. The average time is used to calculate the rate of reaction at that temperature. B. Determination of the reaction order with respect to acetone and hydrogen ion 1. The procedure outline in Part A is repeated using the volumes of reactants given in Table 1. Table 1: Volumes of reactants used in experiments Volume of solution to be used (mL) Experiment Test tube B Test tube A Bromine HCl Acetone Distilled water 1 10 10 10 20 2 15 10 10 15 3 10 20 10 10 4 10 20 15 10 C. Determination of the activation energy of the reaction 1. The procedure outline in Part A is repeated at different temperature (40℃,45℃ and 50℃). 2. The order of the reaction is determined with respect to each of the reactants. 3. The rate equation of this reaction is determined. 4. The rate of reaction at each temperature studied is calculated and determined the activation energy of the reaction from the graph of ln rate vs 1/T. The reasons of graph used to determine the activation energy is ln rate vs 1/T is explained. RESULTS PART A Time taken to become colourless (s) Experiment Temperature (℃) First reading 1 23 1217 Second reading 783 Average 1000 PART B (CONSTANT TEMPERATURE) Time taken to become colourless (s) Experiment Temperature (℃) First reading Second reading Average 1 23 1217 783 1000 2 23 895 3420 2158 3 23 450 308 379 4 23 385 377 381 PART C (CONSTANT VOLUME) Experiment 1 Temperature (℃) Time taken to become colourless (s) 40 95 45 83 50 39 CALCULATIONS PART A & B Calculation of new concentration of reactant Exp 1 : Acetone : Concentration (new) = Concentration of reactant × Volume Total volume = 4.0 M (10 mL) 50 mL = 0.8 M Acetone + Br2 + H+ → Acetone-Br + H+ + Br- Experiment 1 Rate = d [Acetone-Br] = - d [Br2] dt dt Rate = 0.004M 1000 s Rate = 4.000 × 10-6 M s-1 Experiment [Acetone],M [H+],M [Bromine],M Rate, Ms-1 1 0.8 0.2 0.004 4.000×10-6 2 0.8 0.2 0.006 2.780×10-6 3 0.8 0.4 0.004 1.056×10-5 4 1.2 0.4 0.004 1.050×10-5 PART C Determination of order of reaction From equation of reaction, Rate law = k[Acetone]x[Br2]y[H+]z Experiment 3 : k[0.8]x[0.004]y[0.4]z k[0.8]x[0.004]y[0.2]z Experiment 1 = 1.056×10-5 4.000×10-6 2z = 2.64 log 2z = log 2.64 z log 2 = log 2.64 z = log 2.64 log 2 Experiment 2 : z = 1.4 z ≈ 1 k[0.8]x[0.004]y[0.2]z k[0.8]x[0.006]y[0.2]z Experiment 1 = 2.780×10-6 4.000×10-6 (3/2)y = 0.695 log (3/2)y = log 0.695 y log (3/2) = log 0.695 y = log 0.695 log (3/2) Experiment 4 Experiment 3 : y = -0.90 y ≈ -1 k[1.2]x[0.004]y[0.2]z k[0.8]x[0.004]y[0.2]z = 1.050×10-5 1.056×10-5 (3/2)x = 0.994 log (3/2)x = log 0994 x log (3/2) = x = log 0.994 log 0.994 log (3/2) x = -0.015 x ≈ 0 ⸫ Rate law = k[H+][Br2]-1 Temperature (K) 1/T (10-3. K-1) Rate (M s-1) ln Rate 296 3.38 4.00×10-6 -12.43 313 3.19 4.21×10-5 -10.08 318 3.14 4.82×10-5 -9.94 323 3.10 1.03×10-4 ×10-3 -9.18 ln rate vs 1/T 0 3,05 3,1 3,15 3,2 3,25 3,3 3,35 3,4 -2 ln rate -4 -6 -8 3,1; -9,18 3,14; -9,94 3,19; -10,08 -10 3,38; -12,43 -12 -14 1/T ( ×10-3K-1) ln k = -Ea + ln A RT Compared with straight line equation y = mx + c y = ln k m = x = 1/T c = ln A where Ea R Determination of activation energy, Ea y2-y1 Gradient of the graph, m = x2-x1 -12.43-(-10.08) = = (3.38-3.1) K-1 -12.37 K Ea From gradient, m = R The negative value is neglected as it indicates the shape of the graph Ea = mR = 12.37K (8.314 J K-1 mol-1) = 102.84 J mol-1 DISCUSSION The bromination of acetone in acid solution proceeds according to Acetone + Br2 + H+ → Acetone-Br + H+ + BrThe reaction is catalysed by hydrogen ion. The rate is determined by Rate = d [Acetone-Br] dt = - d [Br2] dt because Br2 is the limiting reactant. The rate for this particular reaction at 23°C is 4.00×10-6 M s-1. The rate law is assumed to be of the form Rate law = k[Acetone] x[Br2] y[H+] z where k is the rate constant. The exponents x, y, and z indicate the order of the reaction with respect to acetone, bromine, and hydrogen ion, respectively. From the result and calculation, we can determine the reaction rate at room temperature, the reaction order for each of the reactants and the activation energy. We know the order of each reactant which is 0 for Acetone, - 1 for Bromine and 1 for hydrogen ion. When a partial order is negative, the overall order is usually considered as undefined. In this experiment, although the rate equation found is Rate law = k[H+][Br2]-1 , the rate equation is more complex than that of a simple order reaction. Rate of the reaction is also determined at several temperatures in this experiment. By plotting ln rate vs 1/T, the activation energy is calculated from the graph which is 102.84 J mol-1. We use graph of ln rate against 1/T which corresponds with the Arrhenius equation because it involves the relationship between rate of reaction and temperature. CONCLUSION The rate, order, and activation energy of the bromination of acetone is determined. REFERENCE Atkins, P., & de Paula, J. (2006). Physical Chemistry, Eighth Edition. New York: Oxford University Pres McCormick, J.M., (2003). Bromination of acetone, University of Kansas, http://chemlab.truman.edu/physical-chemistry-bromination-of-acetone Warkentin, J. (2011). Kinetics of bromination of acetone, bromoacetone and 1,1-dibromoacetone, www.researchgate.net