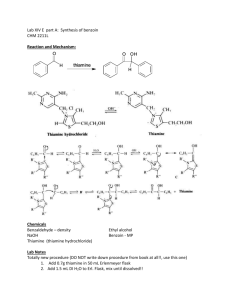
- Experiment 32: The Conversion of Benzaldehyde to Benzilic Acid Fredrick McCorkle Organic Chemistry Lab, CHEM 369 The University of Tennessee, Knoxville Sammy Eni Dr. L. M. Smith Dates Performed: September 19, 26, & October 3, 10, 2014 Date due: October 31st , 2014 Introduction The purpose of this experiment is to observe and perform a multi-step synthesis of benzilic acid, with the starting compound of benzaldehyde. The product of each step was isolated by vacuum filtration, purified through recrystallization, and analyzed by calculating percent yield, determination of melting point (m.p.), Infrared Spectroscopy (IR), and/ or 1H and 13C Nuclear Magnetic Resonance Spectroscopy (NMR). The multi-step synthesis consisted of: First, a condensation reaction of benzaldehyde to benzoin using a thiamine HCl (vitamin B) catalyst wherein the hydrogen is removed from the C2 carbon of the thiazole ring in thiamine HCl, forming an ylide. The ylide carries out a nucleophilic attack upon the carbonyl group of the aldehyde, forming an intermediate that can interact with a new benzaldehyde molecule to form a compound structure. A base takes a proton from the first benzaldehyde, inducing bond changes that result in the formation of benzoin. Reaction 1 The second reaction was that of and oxidation of benzoin to the ketone benzil using a nitric acid oxidizing agent that pulls the hydrogen off an oxygen atom rendering a lone pair that forms a double bond. Reaction 2 The third reaction was a rearrangement of benzil instigated by KOH to form a stable carboxylate salt (potassium benzilate). Acidification through HCl replaces the potassium atom with a hydrogen atom, forming benzilic acid. Reaction 3 Procedures and Observations The experiment began by adding (1.48 grams) thiamine HCl, 2ml of water, 15ml ethanol (90%)(Observation- slightly exothermic upon this addition), and 4.5ml of sodium hydroxide to a 50ml Erlenmeyer flask, swirling between each addition, and on the final addition, swirling the flask until the bright yellow solution turns dull. Then add 4.5ml of benzaldehyde and set aside for a week. Next isolation of the product was conducted by crystallizing the flask in an ice bath 10 minutes, followed by vacuum filtration, using cold water to wash the solution from the solid. This was dried, weighed (5.26 grams), % yield was determined (113%), and the m.p. was determined (120°C). Recrystallization followed to purify the product. 95% Ethanol was used to dissolve the product (at a minimum to preserve the yield), was cooled, and again vacuum filtered. Melting point was again taken (129°C) as well as the weight (4.59 grams) and yield (98.3%). Part B began with adding (2.53 grams) of the product formed in part A (benzoin) to a 25ml round bottom (r.b.) flask along with 12ml of concentrated nitric acid and a stir bar. A water condenser was attached and the flask was heated under a hood in a hot water bath at 60-70°C for 1 hour (when no gases were evolved). The mixture was then poured into a beaker with 40ml of cold water and stirred until a yellow solid formed. This was vacuum filtered with more cold water and dried. The product was weighed (2.8 grams) and the yield determined (111%). Recrystallization followed in the same way. Melting point was then determined (85°C) as well as weight (2.44 grams) and yield (97.4%). Part C began by adding the benzil product (2.1 grams) and 6ml of 95% ethanol to a 25ml r.b. flask with a boiling stone. A reflux was set up and the solution was heated up to the dissolution of the benzil, at which point 5ml of KOH was added dropwise through the top of the condenser and the mixture was allowed to boil for 15 minutes. (Obs.- Mixture initially turned black, then very slight change to dark brown) The mixture was then cooled 5 minutes, added to a small beaker, crystallized with 95% ethanol, and vacuum filtered. The solid was then transferred to a 100ml Erlenmeyer flask containing 60ml of 70°C water and dissolved. To this was added 1.3ml HCl, until the pH reached 2 (checked with litmus paper), the solution was cooled in an ice bath, and the product was collected through vacuum filtration. The weight (1.96 grams), yield (85.9%), and melting point (142°C) were determined. IR spectra were to be taken for each part and 1H & 13C NMR spectra were provided for part C. Mechanism Condensation of Benzaldehyde Oxidation of Benzoin Rearrangement of Benzil Data Reagent Benzaldehyde Benzoin Benzil Benzilic Acid Thiamine HCl 95% EtOH NaOH (aq) HNO3(aq) KOH (aq) HCl (aq) Amount used/ % yield structure 4.68grams C7H6O 4.59g (98.3%) C14H12O2 2.44g (97.4%) (C₆H₅CO)₂ C H O 1.96g (85.9%) 1.48 grams C12H17N4OS ~60mL total CH3CH2OH 4.5mL NaOH 12mL HNO3 5mL KOH 1.3mL HCl 14 12 3 Role Mol. Weight Reactant 106.121 Product/reactant 212.24 Product/reactant 210.23 Product 228.24 reactant 265.35 solvent 46.07 catalyst 39.99 catalyst/solvent 63.01 catalyst 56.1 catalyst 36.46 mp/bp density (g/mL) hazards ~/179°C 1.04 136°C/344°C 1.31 94-96°C/346-348°C 1.23 150-152°C/180°C 1.08 ~ ~ ~/78 0.789 ~ 2.13 corrosive ~/83°C 1.51 corrosive ~ 2.04 corrosive ~ 1.49 corrosive Example calculation: Benzaldehyde to Benzoin theoretical yield & actual yield 4.5mL (1.04g/mL)=4.68g 4.68g/ (106.1g/mol)=.044mol .044mol/ 2= .022mol Benzoin .022(212.24g/mol) = 4.67 grams (Theoretical Yield) 4.59g (recovered)/ 4.67g =98.3% Actual Yield Results and Discussion The multi-step synthesis of benzilic acid was carried out in the way described and the following results were subsequently collected. The % yield was much higher than one would expect, with percentages of 98.3, 97.4, and 85.9, respectively. However, the melting point was on average far from the literature values, being 7°, 9°, and 8° less than the expected pure product values, respectively. Unfortunately no IR spectra are available to my own fault. The results of the 13C NMR spectra for benzilic acid (Figure 1) indicate an alkoxy group (RCH2O-) at 81 ppm, and a (probable) C in an aromatic ring at 127 and 128 ppm and possibly 141 ppm, indicating increased deshielding. The shift at 178 ppm should indicate the carboxylic acid group. The 1H NMR spectrum (Figure 2) displays two sets of Hydrogen signals between 7.3 and 7.5, indicating 2 aryl groups which should be the 2 benzenes in the product. As noted, a higher frequency NMR instrument should have shown a signal between 10-13 ppm: a hydrogen atom bonded to the oxygen in a carboxylic acid. The signal appearing at 2.2 may be the hydrogen in the alcohol group of the molecule. The intermediate structures cannot be confirmed to match the intended products, for no infrared spectra were taken. However, the final product is likely supported from the 1H and 13C NMR spectra despite the melting point being off. The efficiency of the multi-step sequence is very high, evidenced by the high yields, but again tempered by the variance of the melting points. The melting points were likely off due to my own error, specifically in technique of melting point determination. I believe the exact error was heating the sample too quickly, not allowing the thermometer to display the correct temperature of the apparatus. Another source of error may have been the assumption that each intermediate was perfectly dry when weighed, when this may not have been the case, leading to an incorrect determination of % yield. Further error lied in allowing the temp. of the hot water bath in part B to rise above 75°C, even if briefly. Conclusion As stated in the introduction, this was an experiment aimed at successfully synthesizing benzilic acid with the starting compound of benzaldehyde. The product/ intermediate of each reaction sequence was isolated by vacuum filtration, purified by recrystallization, weighed, and analyzed. 1H & 13C NMR spectra were used to confirm the identity of the product, benzilic acid, with the 13C NMR spectrum showing shifts that indicate aromatic rings affected by the deshielding of electron withdrawing groups, a carboxylic acid group and the stretch of a C-O bond, all of which are present in the desired product. The 1H NMR spectrum further confirmed the presence of 2 aryl rings, with signals between 7.3 and 7.5 ppm; typical signals of hydrogens bonded to an aryl ring. The 1H NMR may also support the alcohol group as well as the carboxylic acid group present in benzilic acid. Citation Pavia, D.L., Lampman, G.M., Kriz, G.S., and Engel, R.G. 2005. Introduction to Organic Laboratory Techniques: A Small Scale Approach. 2nd Ed. Thomson Brooks/Cole, Belmont.