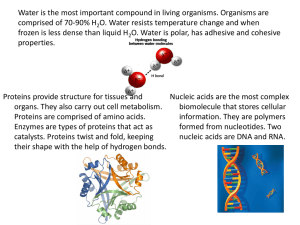
Chapter 1 Macromolecules Macromolecules: Giant Polymers • There are four major types of biological macromolecules: Proteins Carbohydrates Lipids Nucleic Acids • These macromolecules are made the same way in all living things, and are present in all organisms in roughly the same proportions. • An advantage of this biochemical unity is that organisms acquire needed biochemicals by eating other organisms. • Macromolecules are giant polymers. 6 • Polymers are formed by covalent linkages of smaller units called monomers. Condensation and Hydrolysis Reactions • Macromolecules are made from smaller monomers by means of a condensation (or dehydration) reaction in which an OH from one monomer is linked to an H from another monomer. • The reverse reaction, in which polymers are broken back into monomers, is a called a hydrolysis reaction. • Energy must be added to make a polymer; and, released when breaking one. Proteins: Polymers of Amino Acids • Proteins are polymers of amino acids. They are molecules with diverse structures and functions. • Each different type of protein has a characteristic amino acid composition and order. • Proteins range in size from a few amino acids to thousands of them. • Folding is crucial to the function of a protein and is influenced largely by the sequence of amino acids. • An amino acid has four groups attached to a central carbon atom. • Amino acids can be classified based on the characteristics of their R groups. • 5 have charged hydrophilic side chains. • 5 have uncharged hydrophilic (polar) side chains. • 7 have hydrophobic (nonpolar) side chains. • Cysteine has a terminal disulfide (—S—S—). • Glycine has a hydrogen atom as the R group. • Proline has a modified amino group that forms a covalent bond with the R group, forming a ring. • Proteins are synthesized by condensation reactions between the amino group of one amino acid and the carboxyl group of another. This forms a peptide linkage. • Thus, proteins are also called polypeptides. • A dipeptide is two amino acids long; a tripeptide, three; etc. A polypeptide is multiple amino acids long. • There are four levels of protein structure: • primary, secondary, tertiary, and quaternary. • The precise sequence (i.e., order) of amino acids is called its primary structure. • The peptide backbone consists of repeating units of atoms: N—C—C-----N—C—C------N—C—C... • Enormous numbers of different proteins are possible. • A protein’s secondary structure consists of regular, repeated patterns in different regions in the polypeptide chain. • This shape is influenced primarily by hydrogen bonds arising from the amino acid sequence (the primary structure). • The two common secondary structures are: • the a-helix, and; • the b-pleated sheet. The a-helix: • Is a right-handed coil. • Ex: Fibrous proteins such as the keratins found in hair, feathers, and hooves. The b-pleated sheets: • Are formed from peptide regions of a single strand that lie parallel to each other. • Ex: Spider silk. • Tertiary structure: Bending and folding results in a macromolecule with specific threedimensional shape. • The primary determinant of the tertiary structure is the interaction between R groups: Disulfide bonds (see slide) Aggregation of hydrophobic side chains van der Waals forces Ionic bonds Hydrogen bonds • Quaternary structure results from the ways in which multiple (2 or more) polypeptide subunits bind together and interact. • This level of structure adds to the three-dimensional shape of the finished protein. • Hemoglobin is an example of such a protein; it has four subunits. • Shape is crucial to the functioning of some proteins: enzymes need certain surface shapes in order to bind substrates correctly. • Changes in temperature, pH, salt concentrations, and oxidation or reduction conditions can change the shape of proteins. • This loss of a protein’s normal 3D structure (and therefore loss of function) is called denaturation. • Chaperonins are specialized proteins that help keep other proteins from interacting inappropriately with one another. • When a protein fails to fold correctly, serious complications can occur. • Some chaperonins help folding; some prevent folding until the appropriate time. Carbohydrates: Sugars and Sugar Polymers • Carbohydrates are carbon molecules with hydrogen groups and hydroxyl groups H—C—OH • They act as energy storage and transport molecules. • They also serve as “carbon skeletons” for other molecules. • There are four major categories of carbohydrates: • • Monosaccharides are the monomers of carbohydrates; simple sugars. • Disaccharides, which consist of two monosaccharides linked by one covalent bond. • Oligosaccharides, which consist of between 3 and 20 monosaccharides • Polysaccharides, which are composed of hundreds to hundreds of thousands of monosaccharides---starch, glycogen, cellulose. There are four major categories of carbohydrates: • Monosaccharides are the monomers of carbohydrates; simple sugars. • Disaccharides, which consist of two monosaccharides linked by one covalent bond. • Oligosaccharides, which consist of between 3 and 20 monosaccharides • Polysaccharides, which are composed of hundreds to hundreds of thousands of monosaccharides---starch, glycogen, cellulose. • The general formula for a carbohydrate monomer is Cn(H2O)n , maintaining a ratio of 1 carbon to 2 hydrogens to 1 oxygen. • During the polymerization, which is a condensation reaction, water is removed. • Therefore, carbohydrate polymers have ratios of carbon, hydrogen, and oxygen that differ somewhat from the 1:2:1 ratios of the monomers. • Different monosaccharides have different numbers or different arrangements of carbons. Most monosaccharides are isomers. • Triose (3-carbon sugars) include glyceraldehyde. • Tetrose (4-carbon sugars) include erythrose. • Pentoses (5-carbon sugars) include ribose, deoxyribose. • Hexoses (6-carbon sugars) include the structural isomers glucose, fructose, mannose, and galactose. • All cells use glucose (monosaccharide) as their preferred energy source. • Exists as a straight chain or ring form. • Ring is more common—it is more stable. • Monosaccharides bind together in condensation reactions to form glycosidic linkages. Glycosidic linkages can be α or β. • Disaccharides have just one glycosidic linkage: sucrose, lactose, maltose, cellobiose. • Oligosaccharides contain more than two monosaccharides. • • Often covalently bonded to proteins and lipids on cell surfaces and act as recognition signals. The human ABO blood types owe their specificity to oligosaccharide chains. Polysaccharides are giant polymers of monosaccharides connected by glycosidic linkages. Starch: storage of glucose in plants Glycogen: storage of glucose in animals Cellulose (i.e., fiber): very stable, good for structural components in plants. Animals have enzymes that can hydrolyze α-glycosidic links, but not β-links. Therefore, fiber is not digestible. • Carbohydrates can be modified by the addition of functional groups: Phosphate added to one or more hydroxyl (—OH) sites creates a sugar phosphate, such as fructose 1,6-bisphosphate. Amino groups can be substituted for —OH groups, making amino sugars, such as glucosamine and galactosamine. Lipids: Water Insoluble Molecules • Lipids are insoluble in water. • This insolubility results from the many nonpolar covalent bonds of hydrogen and carbon in lipids. • Lipids aggregate away from water, which is polar, and are attracted to each other via weak, but additive, van der Waals forces. • Lipids are the only group of macromolecules that does not consist of polymers. • Lipids are, thus, not made up of a particular monomer, but smaller “fatty” molecules such as fatty acids and glycerol. Fats and oils • Fats and oils store energy. • Chemically, fats and oils are triglycerides, composed of three fatty acid molecules and one glycerol molecule. Glycerol is a three-carbon molecule with three hydroxyl (—OH) groups, one for each carbon. Fatty acids are long chains of hydrocarbons with a carboxyl group (—COOH) at one end. • Saturated fatty acids have only single carbon-to-carbon bonds (i.e., no double bonds) and are said to be saturated with hydrogens. • Saturated fatty acids are rigid and straight, and solid at room temperature (i.e., fat). Animal fats are saturated. • Unsaturated fatty acids have at least one double-bonded carbon in one of the chains — the chain is not completely saturated with hydrogen atoms. • monounsaturated: one double bond • polyunsaturated: more than one double bond • The double bonds cause “kinks” that prevent easy packing. • Thus, they are liquid at room temperature (i.e., oil). Plants commonly have unsaturated fatty acids. Phospholipids • Phospholipids have two hydrophobic fatty acid tails and one hydrophilic phosphate group attached to the glycerol. • As a result, phospholipids orient themselves so that the phosphate group faces water and the tail faces away. • Phospholipids are amphipathic: they have a hydrophilic and a hydrophobic end • In aqueous environments, these lipids form bilayers, with heads facing outward, tails facing inward. Cell membranes are structured this way. Carotenoids & Chlorophylls • These are light-absorbing pigments found mostly in plants. • The carotenoid b-carotene is a plant pigment used to trap light in photosynthesis. • In animals, b-carotene can be broken into two molecules of vitamin A. Steroids • Steroids are signaling molecules/hormones. • Steroids are organic compounds with a series of fused rings. • The steroid cholesterol is a common part of cell membranes, especially in animals. • Cholesterol also is an initial substrate for synthesis of the hormones’ testosterone and estrogen. Fat-soluble vitamins • Some lipids are vitamins: small organic molecules essential to health, not synthesized by the body, so must be acquired from diet. • The lipid-soluble vitamins are: A, D, E, and K. • All others (B’s and C) are water-soluble. Waxes • Waxes are highly nonpolar molecules consisting of saturated long fatty acids bonded to long fatty alcohols via an ester linkage. • A fatty alcohol is similar to a fatty acid, except for the last carbon, which has an —OH group instead of a —COOH group. • Waxy coatings repel water and prevent water loss from structures such as hair/fur, feathers, and leaves.