Synthesis of ferrites
advertisement

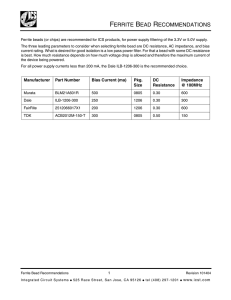
ISSN 0020-1685, Inorganic Materials, 2009, Vol. 45, No. 11, pp. 1309–1313. © Pleiades Publishing, Ltd., 2009. Original Russian Text © M.I. Ivanovskaya, A.I. Tolstik, V.V. Pan’kov, 2009, published in Neorganicheskie Materialy, 2009, Vol. 45, No. 11, pp. 1398–1403. Synthesis of Zn0.5Mn0.5Fe2O4 by Low-Temperature Spray Pyrolysis M. I. Ivanovskayaa, A. I. Tolstikb, and V. V. Pan’kovb a Research Institute of Physicochemical Problems, Belarussian State University, Leningradskaya ul. 14, Minsk, 220030 Belarus b Belarussian State University, Leningradskaya ul. 14, Minsk, 220030 Belarus e-mail: ivanovskaya@bsu.by Received September 15, 2008; in final form, January 12, 2009 Abstract—The effect of synthesis temperature on the structural perfection of the Zn0.5Mn0.5Fe2O4 ferrite synthesized via spray pyrolysis of a solution of Zn(II), Mn(II), and Fe(III) nitrates has been studied using X-ray diffraction, scanning electron microscopy, and IR spectroscopy. The material obtained at 650°C is shown to have a nanocrystalline structure. IR spectroscopy results indicate that the synthesized Zn0.5Mn0.5Fe2O4 spinel ferrite is highly homogeneous in composition and structure. DOI: 10.1134/S0020168509110223 INTRODUCTION An important point in the synthesis of magnetic materials based on Zn–Mn ferrite is the ability to obtain a phase-pure spinel-structure product uniform in composition and structure. The formation of phase-pure Zn–Mn ferrite and its magnetic properties are adversely influenced by the oxidation of Mn2+ to Mn3+, which occurs most rapidly at temperatures above 900°ë. One way of preventing the Mn2+ Mn3+ process is by reducing the ferrite synthesis temperature. The temperature of ferrite formation can be reduced by utilizing nanotechnology instead of conventional ceramic processing techniques (sintering of oxides). The use of nanophase precursors accelerates diffusion of reactants and ensures the formation of highly homogeneous solid solutions. In particular, as shown by Sviridov et al. [1] highly homogeneous, fine-particle zinc ferrite can be prepared through hydroxide coprecipitation. Zn(II) and Fe(III) hydroxide coprecipitation products can be converted to zinc ferrite by aging the precipitate at 100°ë. The formation of manganese ferrite is known to be less energetically favorable than that of zinc ferrite: the activation energy for ferrite formation is 410.5 kJ/mol in the ZnO–Fe2O3 system and 754.1 kJ/mol in the MnO–Fe2O3 system. As reported by Novosadova et al. [2], when hydroxide coprecipitation products with a Zn : Mn ratio of 1 : 1 are converted to the Zn0.5Mn0.5Fe2O4 phase at 100°ë, the yield is about 65%. Therefore, the synthesis of Zn–Mn ferrite requires a higher temperature in comparison with ZnFe2O4, independent of the procedure. However, as mentioned above, the oxidation of Mn2+ to Mn3+ is more likely at higher temperatures. This process may be accompanied by the reduction of Fe3+ to Fe2+ and cation redistribution over the tetrahedral and octahedral sites of the spinel structure. The cation distribution in the spinel structure of Zn–Mn ferrite plays a key role in determining its magnetic properties. There are reports that, during solid-state synthesis from oxides, the spinel structure of Zn–Mn ferrite begins to form at 600°ë, and the process involves the formation of intermediate oxide phases. The first to form is ZnFe2O4 with an excess of uncombined Fe2O3 because the Zn2+ ion is rather mobile and always occupies the tetrahedral site in the spinel structure, with no magnetic hindrances during diffusion. Mn2+ diffuses into the structure of ZnFe2O4 · Fe2O3 at higher temperatures [3]. The optimal heat treatment temperature, which ensures the required magnetic performance of ferrites, is 1000–1200°ë. The synthesis temperature of Zn–Mn ferrite can be reduced by using spray pyrolysis. This method has been shown to be effective in the synthesis of magnetic materials based on barium hexaferrite, BaFe12O19 [4]. Owing to the small reaction volume, the hexaferrite structure can be obtained by spray pyrolysis of nitrate solutions without intermediate oxide phases, which allows the synthesis temperature to be reduced and enables the preparation of nanocrystalline material. Previous results [4] suggest that the synthesis temperature of spinel ferrites can also be reduced. In this paper, we describe the preparation of the Zn0.5Mn0.5Fe2O4 ferrite by low-temperature spray 1309 1310 IVANOVSKAYA, TOLSTIK OH Powder XRD patterns were collected on a DRON2.0 diffractometer with Coäα radiation (λ = 0.178896 nm, Ni monochromator) in the angular range 2θ = 20°– 80°. The results were analyzed by standard procedures using JCPDS PDF data. Mass magnetization was measured as a function of temperature by the Faraday method in a magnetic field H = 0.86 T during heating and cooling in the range 77– 700 K. NOx 6 5 Transmission 4 3 2 1045 1277 1389 1471 1 1800 1700 1600 1500 1400 1300 1200 1100 1000 Wavenumber, cm–1 Fig. 1. Portions of the IR spectra of samples obtained by thermal decomposition of an aqueous solution of Zn(II), Mn(II), and Fe(III) nitrates in air at (1) 100, (2) 200, (3) 300, (4) 400, (5) 500, and (6) 650°C. pyrolysis of a mixture of zinc, manganese, and iron nitrate solutions. EXPERIMENTAL To synthesize Zn–Mn ferrite by spray pyrolysis, we used a solution of Zn(II), Mn(II), and Fe(III) nitrates with a concentration corresponding to 0.25 M Zn0.5Mn0.5Fe2O4. The solution was prepared by mixing solutions of the individual metals in the required proportions. Ferrite samples were prepared in a tubular furnace through which droplets of the solution dispersed by an ultrasonic atomizer were propelled by air. The structure of the samples was examined by X-ray diffraction (XRD), scanning electron microscopy (SEM) on a LEO 1420 instrument, and IR spectroscopy. IR spectra were taken on a Nicolet Avatar 330 spectrometer in diffuse reflection mode (without KBr) in the range 400–4000 cm–1. A small amount of the powder was applied to a steel substrate. RESULTS AND DISCUSSION Synthesis temperature. To select the synthesis temperature sufficient for the synthesis of the ferrite, an aqueous solution of Zn(II), Mn(II), and Fe(III) nitrates with the same composition as was used in spray pyrolysis was heated in air, and the decomposition products were characterized by IR spectroscopy. Analysis of the IR spectra allowed us to determine the temperature range where the nitrate/nitrite residues were removed. The nitrates of the metals in question are known to melt at t ≤ 50°ë. When heated to 100°ë, Zn(II), Mn(II), and Fe(III) nitrates have the form of viscous paste, and their IR spectra show strong absorption bands at 1471, 1389, 1277, and 1045 cm–1, due to bond vibrations in – the NO 3 . ion. According to earlier results, the stretching frequen– – cies of the NO 3 and NO 2 radical anions in inorganic compounds lie in the range 970–1500 cm–1. Figure 1 illustrates the temperature effect on the IR spectra of the decomposition products in the stretching region of the nitrate and nitrite ions. It follows from these data that both the number of absorption bands and their intensity decrease with increasing temperature. The concentration of nitrogen-containing radical anions is low after heating at 400°ë, and there are no such anions after heating at 500–650°ë. The weak feature at 1347 cm–1 in the spectrum obtained after heating at 500°ë may be due not to residual NO2 groups but to CO2 adsorption on the surface of the forming oxide structure. At the same time, there is evidence that ther– mal dehydration of hydroxides in the presence of NO 3 • may lead to stabilization of NO 2 , radical anions, which cause partial amorphization of the resulting oxides [5]. • NO 2 radical anions were detected by ESR in highly dispersed Al2O3, MÓO3, In2O3, and SnO2 prepared by sol–gel processing from colloidal suspensions stabi– lized with NO 3 anions [5, 6]. It follows from our IR spectroscopy data that the decomposition of the nitrate/nitrite residues during heating of a mixture of zinc, manganese, and iron nitrates reaches completion in the range 500–650°ë. In INORGANIC MATERIALS Vol. 45 No. 11 2009 SYNTHESIS OF Zn0.5Mn0.5Fe2O4 BY LOW-TEMPERATURE SPRAY PYROLYSIS 1311 2 1 75 70 65 60 55 50 45 40 35 2θ, deg Zn0.5Mn0.5Fe2O4 Mn2O3 ZnO α-Fe2O3 Fig. 2. XRD patterns of the Zn–Mn ferrite samples synthesized at (1) 450 and (2) 650°C. (a) (b) 2 828 949 1038 1205 1162 1122 1082 1040 1 560 430 1402 1633 1331 1038 Transmission 1 3408 Transmission 2 4000 3000 2000 1000 Wavenumber, cm–1 0 1400 1200 1000 800 600 Wavenumber, cm–1 Fig. 3. IR spectra of the Zn–Mn ferrite samples synthesized at (1) 450 and (2) 650°C: (a) 400–4000 cm–1, (b) 500–1500 cm–1. view of this, two temperatures were chosen for the synthesis of Zn–Mn ferrite by spray pyrolysis: 450 and 650°ë. The lower synthesis temperature (450°ë) was intended to ascertain whether dehydration, nitrate decomposition, and ferrite crystallization in the system under investigation occur more rapidly when they are localized in a microvolume (in a droplet). We took into account the Mössbauer results reported by Sviridov et al. [7], which demonstrate that, in highly dispersed Fe(III) and M(II) (M = Zn, Cu, Ni) hydroxide coprecipitation products, radical structural changes resulting in ferrite formation begin and 400°ë and reach completion at 500°ë. Phase composition, particle size, and crystal structure. The XRD pattern of the sample prepared at 450°ë shows one, broad line, which can be identified as the 311 reflection from the spinel ferrite phase (JCPDS, INORGANIC MATERIALS Vol. 45 No. 11 2009 no. 10-0467) (Fig. 2). This indicates that the temperature 450°ë is too low for the formation of the Zn0.5Mn0.5Fe2O4 spinel. The crystallite size is 4–5 nm. In addition, the XRD pattern shows a weak halo in the 2θ range of the strongest reflections from ZnO (39.30°), α-Fe2O3 (38.69°), and Mn2O3 (38.40°), which may be interpreted as evidence that the sample contains small amounts of these oxides in a highly dispersed state. The same is evidenced by IR spectroscopy data: the broad absorption band in the frequency range characteristic of M–O (M = Fe, Zn, Mn) bond vibrations (500 cm–1) suggests that the ferrite phase is poorly ordered and that the sample may contain binary oxides (Fig. 3). The features at 828, 949, and 1038 cm–1, characteristic of the Zn–O–H, Mn–O–H, and Fe–O–H bending modes in the corresponding oxides, lend support to the assumption that the sample contains ZnO, MnO, and Fe2O3. 1312 (‡) IVANOVSKAYA, TOLSTIK 2 µm 2 µm (b) Fig. 4. SEM micrographs of Zn–Mn ferrite particles synthesized at (a) 450 and (b) 650°C. The strong, broad absorption band at 3408 cm–1 attests to a large amount of adsorbed water, which is rather typical of highly dispersed α-Fe2O3. The feature at 1633 cm–1, due to the O–H bending mode, indicates that not all of the OH groups were removed from the material, which is also typical of the various forms of FeOOH present as reaction intermediates when dehydroxylation of the oxide system is incomplete. The strong absorption bands at 1331 and 1402 cm–1 are due to the residual nitrate and nitrite ions in the sample synthesized at 450°ë. The above experimental data indicate that this temperature is too low for the synthesis of phase-pure spinel-structure Zn0.5Mn0.5Fe2O4 by spray pyrolysis. The forming fine-particle material seems to be a mixture of poorly crystallized (ZnMn)xFe2O4, Fe2O3, ZnO, – and MnOx, with large amounts of H2O, NO x - , and OH groups. According to SEM results (Fig. 4), the sample consists of regularly shaped, spherical particles. The surface morphology of the particles is typical of amorphous or glassy materials. The particle diameter d ranges from 150 nm to 1.6 µm. Most of the particles have d = 650–800 nm. It seems likely that these are aggregates of finer particles, given that the crystallite size determined by XRD is almost two orders of magnitude smaller than the average diameter of the spherical particles. The XRD pattern of the sample prepared at 650°ë shows broad lines attributable to a spinel ferrite phase (Fig. 2). No other phases were detected by XRD. The crystallite size is 7–8 nm. The unit-cell parameter of this sample (aav = 8.470 Å, ‡511 = 8.443 Å) is smaller than those reported for high-temperature Zn–Mn ferrite samples of different compositions (8.459–8.472 Å). It is known that, for a given composition, the unit-cell parameter of Zn–Mn ferrites increases with calcination temperature. IR spectroscopy results confirm that this sample has the spinel structure with a rather uniform cation distribution. In the region characteristic of Fe–O bond vibrations in the spinel structure, its IR spectrum shows two symmetric absorption bands, centered at 430 and 560 cm–1, which are assignable to Fe–O–Zn and Fe–O–Mn combined bond vibrations (Fig. 3). The shift of the IR absorption bands characteristic of the Fe–O stretching mode in Fe3O4 to lower frequencies 430 cm–1) indicates the (580 560 cm–1, 440 presence of Zn2+ and Mn2+ in the spinel structure [8, 9]. The symmetric shape of these bands suggests that the Zn0.5Mn0.5Fe2O4 ferrite has a homogeneous spinel structure and that the Zn2+ and Mn2+ cations are evenly distributed over its sites. The chemical homogeneity of the Zn–Mn spinel ferrite is also evidenced by the absence of the absorption band characteristic of Mn–O–H bends in Mn2O3 (949 cm–1), the reduced intensity and shift (836 828 cm–1) of the Zn−O–H bending band, and the symmetric splitting of the absorption band characteristic of Fe–O–H bends in the oxide into five equally spaced (∆ 40 cm–1) components (1040, 1082, 1122, 1162, and 1205 cm–1). The splitting of the Fe–O–H bending band may be due to the reduction of the symmetry of the octahedral oxygen coordination of Fe(III) in the structure of the ferrite as a result of the accommodation of the large-sized cations Zn2+ and Mn2+ in the tetrahedral site. From the temperature dependence of mass magnetization for the Zn–Mn ferrite sample synthesized at 650°ë (Fig. 5), its Curie temperature TC was determined INORGANIC MATERIALS Vol. 45 No. 11 2009 SYNTHESIS OF Zn0.5Mn0.5Fe2O4 BY LOW-TEMPERATURE SPRAY PYROLYSIS σ, A m2/kg 22 1313 Heating Cooling Second heating 20 18 16 14 12 10 8 TC = 375–380 K 6 4 2 0 0 100 200 300 400 500 600 700 800 Temperature, K Fig. 5. Temperature dependence of mass magnetization for the Zn–Mn ferrite sample synthesized at 650°C. to be 375–380 K. The shape of the σ (T) curve and the value of TC confirm the formation of a phase-pure ferrite and indicate that the material contains no uncombined iron oxide [10]. 3. Pankov, V.V., Interaction of (Mn,Zn)O Solid Solution with Fe2O3 As Intermediate Stage of Formation of Mn–Zn Ferrites, Ceram. Int., 1988, vol. 20, pp. 87–91. CONCLUSIONS Nanocrystalline Zn0.5Mn0.5Fe2O4 with the spinel structure was synthesized via spray pyrolysis of a solution of Zn(II), Mn(II), and Fe(III) nitrates at 650°C. Single-phase material with the spinel structure and a unit-cell parameter ‡ = 8.443–8.470 Å was shown to form at 650°ë. The ferrite particles thus produced have a regular spherical shape and range in diameter from 650 to 800 nm, which is typical of spray pyrolysis because structure formation processes take place in microscopic droplets of the starting solution. IR spectroscopy results indicate that the zinc and manganese cations are evenly distributed in the structure of the synthesized ferrite. The crystallite size is 7–8 nm as determined by XRD. REFERENCES 1. Sviridov, V.V., Adamovich, T.I., Kuntsevich, N.I., and Lobanok, A.D., Formation of Zinc Ferrite from Zn(OH)2– Fe(OH)3 Coprecipitated Hydroxides, in Fizicheskie svoistva ferritov (Physical Properties of Ferrites), Minsk: Nauka i Tekhnika, 1967, p. 95. 2. Novosadova, E.B., Drigibka, Ya.G., Pashkova, E.V., et al., Polycrystalline and Single-Crystal Manganese Zinc Ferrite INORGANIC MATERIALS Vol. 45 Materials, in Marganetssoderzhashchie ferrity: Sintez i fiziko-khimicheskie svoistva (Manganese-Containing Ferrites: Synthesis and Physicochemical Properties), Moscow: Nauka, 1986, p. 18. No. 11 2009 4. Pan’kov, V.V., Application of Spray Pyrolysis in the Preparation of Functional Materials, Vestn. Beloruss. Gos. Univ., Ser. 2, 2007, no. 2, pp. 3–13. 5. Kravchuk, L.S., Ugolev, I.I., Kozlov, N.S., et al., Texture and Paramagnetic Properties of Amorphous Alumina, React. Kinet. Catal. Lett., 1984, vol. 25, nos. 1–2, pp. 55–58. 6. Ivanovskaya, M., Ceramic and Film Metaloxide Sensors Obtained by Sol–Gel Method: Structural Features and GasSensitive Properties, Electron. Technol., 2001, vol. 33, nos. 1–2, pp. 108–112. 7. Sviridov, V.V., Belozerskii, G.N., Baikov, M.V., et al., Mössbauer Study of Ferrite Formation from Coprecipitated Hydroxides, Kinet. Katal., 1974, vol. 15, no. 4, pp. 929–934. 8. Gillot, B., Jemmali, F., and Rousset, A., Infrared Studies on the Behavior in Oxygen of Cobalt-Substituted Magnetites: Comparison with Zinc-Substituted Magnetites, J. Solid State Chem., 1983, vol. 50, pp. 138–145. 9. Gillot, B., Benloucif, R.M., and Rousset, A., A Study of Infrared Absorption in the Oxidation of Zinc-Substituted Magnetites to Defect Phase and Hematite, J. Solid State Chem., 1981, vol. 39, pp. 329–336. 10. Bashkirov, Sh.Sh., Electronic Properties and Magnetic Microstructure of Manganese Zinc Spinel Ferrites, in Marganetssoderzhashchie ferrity: Sintez i fiziko-khimicheskie svoistva (Manganese-Containing Ferrites: Synthesis and Physicochemical Properties), Moscow: Nauka, 1986, p. 52.