CHAPTER 14 SUMMARY
advertisement

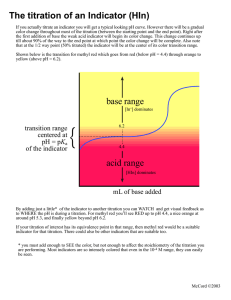
MKG BSMT-IB CHAPTER 14: NEUTRALIZATION TITRATION Used to determine the amounts of acids and bases Used to monitor the progress of reactions that produce or consume hydrogen ions. Depends on a chemical reaction of the analyte with a standard reagent TYPES OF ACID/BASE TITRATIONS: Titration of STRONG ACID ( HCl, H2SO4) with STRONG BASE (NaOH) Titration of WEAK ACID (acetic acid, lactic acid) with STRONG BASE Titration of WEAK BASES (sodium cyanide, sodium salicylate) with strong acid Methods of determining/ locate the end point: Use of a chemical indicator Instrumental method STANDARD SOLUTIONS The standard solutions used in neutralization titrations : STRONG ACIDS (HCl, HClO4, H2SO4) STRONG BASES (NaOH, KOH) o because these substances react more completely with an analyte o and Produce sharper endpoints Standard solution of acids are prepared by diluting CONCENTRATED: Hydrochloric acid Perchloric acid Sulfuric acid Nitric acid is seldom used because its oxidizing properties offer the potential for undesirable side reactions Hot concentrated perchloric and sulfuric acids are potent oxidizing agents and are very hazardous Cold dilute solutions of these reagents are safe to use in analytical laboratory without any precautions other than eye protection Standard solutions of bases are prepared from SOLID: Sodium hydroxide Potassium hydroxide (occasionally) barium hydroxides ACID/BASE INDICATORS Naturally occurring or synthetic compounds that exhibit colors depending on the pH of the solutions in which they are dissolved Indicate alkalinity or acidity It is a weak organic acid or weak organic base whose undissociated form differs in color from its conjugate In the dissociation of an acid-type indicator, the hydronium ion is proportional to the ratio of the concentration of the acid form to the concentration of the base form of the indicator, which in turn controls the color of the solution. The human eye is not very sensitive to color diffrences in a solution containing a mixture of HIn and In-, especially when the ration is greater than 10 or smaller than 0.1. HIn, exhibits its pure acid color when the ratio is equal to or greater than 10 HIn, exhibits its base color when the ratio equal to or lesser than 0.1 The range of hydronium ion concentration for full acid color [𝐻3 𝑂+ ] = 10𝐾𝑎 The range of hydronium ion concentration for full base color [𝐻3 𝑂 + ] = 0.1𝐾𝑎 Indicator pH range 𝑝𝐻 (𝑎𝑐𝑖𝑑 𝑐𝑜𝑙𝑜𝑟) = 𝑝𝐾𝑎 + 1 𝑝𝐻 (𝑏𝑎𝑠𝑒 𝑐𝑜𝑙𝑜𝑟) = 𝑝𝐾𝑎 − 1 𝑖𝑛𝑑𝑖𝑐𝑎𝑡𝑜𝑟 𝑝𝐻 𝑟𝑎𝑛𝑔𝑒 = 𝑝𝐾𝑎 ± 1 TITRATION ERRORS WITH ACID/BASE INDICATORS Two types of errors: 1. Determinate errors occurs when the pH at which the indicator changes differ from the pH at equivalence point can be minimized by choosing the indicator carefully or by making a blank correction 2. Indeterminate error MKG BSMT-IB originates from the limited ability of the human eye to distinguish reproducibly the intermediate color of the indicator the magnitude of this error depends on: -change in pH per milliliter of reagent at the equivalence point -concentration of the indicator -Sensitivity of the eye to the two indicator colors The visual uncertainty with an acid/base indicator is in the range of ±0.5 𝑡𝑜 1𝑝𝐻 𝑢𝑛𝑖𝑡 This uncertainty can be decreased to as little as ±1𝑝𝐻 𝑢𝑛𝑖𝑡 by matching the color of the solution being titrated with that of a reference standard containing a similar amount of indicator at appropriate pH Variables that influence the behavior of indicators Temperature Ionic strength of the medium Presence of organic solvents and colloidal particles - The last two can cause the transition range to shift by one or more pH units Common Acid/Base Indicators For solutions of strong base, the concentration of OH- is equal to the analytical concentration of the bases. TITRATION OF A STRONG BASE WITH a STRONG ACID Three types of calculations must be done to construct the hypothetical curve for titrating a solution of a strong acid with a strong base. Stages of titration: 1. Preequivalence 2. Equivalence 3. Postequivalence In preequivalence stage: compute the concentration of the acid from its starting stage concentration and the amount of base added At equivalence point: the hydronium and hydroxide ions are present in equal concentrations. The hydronium ion concentration can be calculated directly from the ion-product constant for water Kw In postequivalence stage: the analytical concentration of the excess base is computed, and the hydroxide ion concentration is assumed to be equal or to a multiple of the analytical concentration 𝑝𝐾𝑤 = 𝑝𝐻 + 𝑝𝑂𝐻 −𝑙𝑜𝑔10−14 = 𝑝𝐻 + 𝑝𝑂𝐻 Choosing an indicator The selection of an indicator is not critical when the reagent concentration is approximately 0.1M Bromocresol green is unsuited for a titration involving the 0.001 M reagent because the color change occurs over a 5ml range well before the equivalence point. Phenolphtalein is subject to similar objections TITRATION OF STRONG ACIDS and BASES Sources of hydronium ions in aqueous solution of a strong acid: Reaction of the acid with water Dissociation of water itself For solutions of strong acid that are more concentrated than about 1x10-6 M, at equilibrium the concentration of H3O+ is equal to the analytical concentration of the acid Only bromothymol blue provides a satisfactory end point with a minimal systematic error in the titration of 0.001M NaOH TITRATION OF A STRONG BASE WITH a STRONG ACID Titration curves are calculated in a similar way to those for strong acids At preequivalence, the solution is basic At equivalence, solution is neutral MKG BSMT-IB At postequivalence the solution becomes acidic and the hydronium ion concentration is equal to the analytical concentration of the excess strong acid 𝑝𝐻 = 𝑝𝐾𝑎 At the half-titration point in a weak-base titration, [𝑂𝐻 − ] = 𝐾𝑏 𝑝𝑂𝐻 = 𝑝𝐾𝑏 TITRATION CURVES FOR WEAK ACIDS Four distinctly different types of calculations are needed to compute values for a weak acid (or weak base) titration curve: 1. At the beginning, the solution contains only a weak acid or a weak base, and the pH is calculated from the concentration of that solute and its dissociation constant. 2. After various increments of titrant have been added (up to, but not including, the equivalence point), the solution consists of a series of buffers. The pH of each buffer can be calculated from the analytical concentrations of the conjugate base or acid and the concentrations of the weak acid or base that remains. 3. At the equivalence point, the solution contains only the conjugate of the weak acid or base being titrated (that is, a salt), and the pH is calculated from the concentration of this product. 4. Beyond the equivalence point, the excess of strong acid or base titrant suppresses the acidic or basic character of the reaction product to such an extent that the pH is governed largely by the concentration of the excess titrant Titration curves for strong and weak acids are identical just slightly beyond the equivalence point. The same is true for strong and weak bases The pH at equivalence point in the titration of a weak acid with a strong base is greater than 7. The solution is basic. A solution of the salt of a weak acid is always basic Half-titration points- used to determine the dissociation constants At the half-titration point in a weak-acid titration, [𝐻3 𝑂 + ] = 𝐾𝑎 *recall 𝐾𝑏 = 𝐾𝑤/𝐾𝑎 The effect of concentration - - - - In calculating the values for more dilute acids, a solution of a quadratic equation is necessary for each point of the curve until after the equivalence point In postequivalence point region, the excess OHpredominates, simple calculatins can be used. The initial pH values are higher and the equivalence-point pH is lower for the more dilute solution. At intermediate titrant volumes the pH values differ only slightly because of the buffering action of weak acids that is present in the regions. The change in [OH] in the vicinity of the equivalence point becomes smaller with lower analyte and reagent concentrations. Effect of Reaction Completeness The pH change in the equivalence-point region becomes smaller as the acid becomes weaker ( reaction between the acid and the base becomes less complete) Choosing and indicator The choice of indicator is more limited for the titration of a weak acid Bromecresol green is unsuited for titration of 0.1000M of weak acid. Bromothymol blue does not work either because of its full color change occurs over a range of titration volume from about 47 ml to 50 ml of 0.1000M base Phenolphtalein, provides a sharp end point with a minimal titration error. Using an indicator with a transition range between phenolphthalein and of bromothymol blue in conjuction with a suitable color MKG BSMT-IB comparison standard makes it possible to establish an endpoint. When titrating a weak acid use an indicator with a mostly basic transition range TITRATION CURVES FOR WEAK BASES Calculations needed to draw the titration curve for weak base are analogous to those of weak acid When titrating a weak base use an indicator with a mostly acidic transition range. THE COMPOSITION OF SOLUTIONS DURING ACID/BASE TITRATIONS The changes in composition during titration can be visualized by plotting the relative equilibrium concentration of the weak acid as well as the relative equilibrium concentration of the conjugate base as functions of the pH of solution.