Enthalpy & Hess's Law Worksheet: Chemistry Problems
advertisement
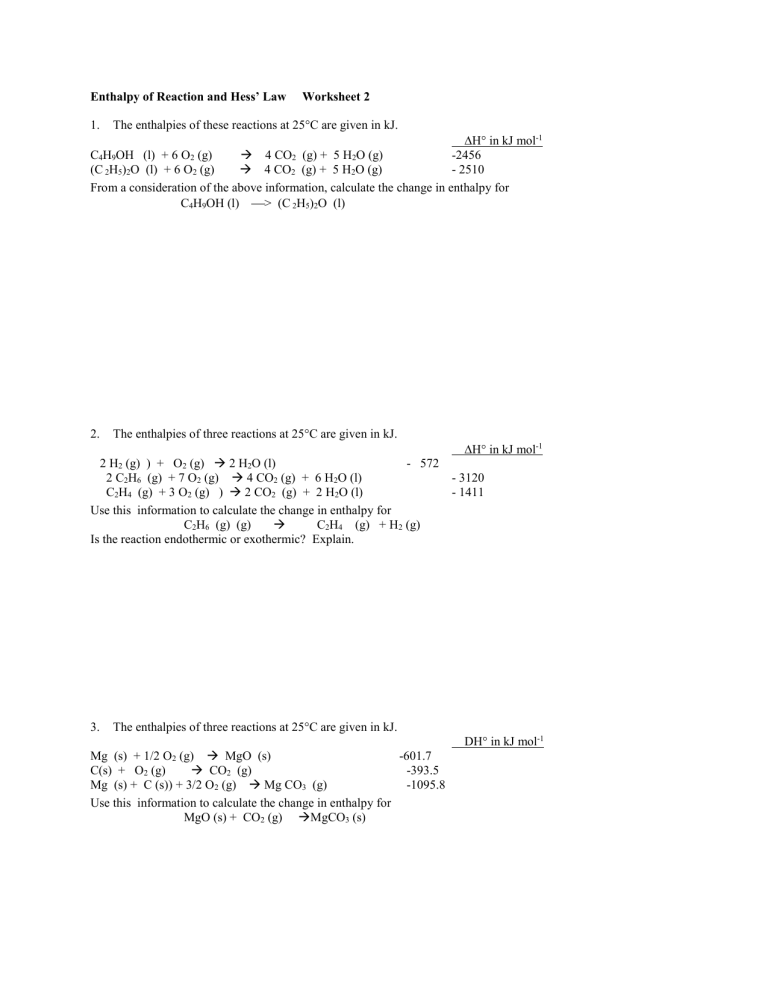
Enthalpy of Reaction and Hess’ Law 1. Worksheet 2 The enthalpies of these reactions at 25°C are given in kJ. H° in kJ mol-1 C4H9OH (l) + 6 O2 (g) 4 CO2 (g) + 5 H2O (g) -2456 (C 2H5)2O (l) + 6 O2 (g) 4 CO2 (g) + 5 H2O (g) - 2510 From a consideration of the above information, calculate the change in enthalpy for C4H9OH (l) > (C 2H5)2O (l) 2. The enthalpies of three reactions at 25°C are given in kJ. H° in kJ mol-1 2 H2 (g) ) + O2 (g) 2 H2O (l) - 572 2 C2H6 (g) + 7 O2 (g) 4 CO2 (g) + 6 H2O (l) C2H4 (g) + 3 O2 (g) ) 2 CO2 (g) + 2 H2O (l) Use this information to calculate the change in enthalpy for C2H6 (g) (g) C2H4 (g) + H2 (g) Is the reaction endothermic or exothermic? Explain. 3. - 3120 - 1411 The enthalpies of three reactions at 25°C are given in kJ. DH° in kJ mol-1 Mg (s) + 1/2 O2 (g) MgO (s) -601.7 C(s) + O2 (g) CO2 (g) -393.5 Mg (s) + C (s)) + 3/2 O2 (g) Mg CO3 (g) -1095.8 Use this information to calculate the change in enthalpy for MgO (s) + CO2 (g) MgCO3 (s) 4. The enthalpies of three reactions at 25°C are given in kJ. DH° in kJ mol-1 C(s) + 1/2 O2 (g) CO (g) C(s) + O2 (g) CO2 (g) CO (g) + 1/2 O2 (g) CO2 (g) H2 (g) ) + 1/2 O2 (g) H2O (g) -241.8 Use this information to calculate the change in enthalpy for C(s) + H2O (g) CO (g) + H2 (g) 5. - 110.5 - 393.5 -283.0 The enthalpies of three reactions at 25°C are given in kJ. H° in kJ mol-1 N2 (g) + 2O2 (g) 2 NO2 (g) + 33.2 2 NO (g) + O2 (g) 2 NO2 (g) - 57.1 From a consideration of the above information, calculate the change in enthalpy for N2 (g) + O2 (g) 2 NO (g)



